Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use
- PMID: 23727937
- PMCID: PMC3709753
- DOI: 10.3390/ijms140611713
Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use
Abstract
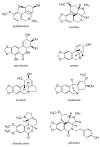
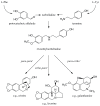
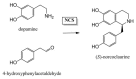
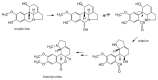
The alkaloids characteristically produced by the subfamily Amaryllidoideae of the Amaryllidaceae, bulbous plant species that include well know genera such as Narcissus (daffodils) and Galanthus (snowdrops), are a source of new pharmaceutical compounds. Presently, only the Amaryllidaceae alkaloid galanthamine, an acetylcholinesterase inhibitor used to treat symptoms of Alzheimer's disease, is produced commercially as a drug from cultivated plants. However, several Amaryllidaceae alkaloids have shown great promise as anti-cancer drugs, but their further clinical development is restricted by their limited commercial availability. Amaryllidaceae species have a long history of cultivation and breeding as ornamental bulbs, and phytochemical research has focussed on the diversity in alkaloid content and composition. In contrast to the available pharmacological and phytochemical data, ecological, physiological and molecular aspects of the Amaryllidaceae and their alkaloids are much less explored and the identity of the alkaloid biosynthetic genes is presently unknown. An improved molecular understanding of Amaryllidaceae alkaloid biosynthesis would greatly benefit the rational design of breeding programs to produce cultivars optimised for the production of pharmaceutical compounds and enable biotechnology based approaches.
Figures

References
-
- Ziegler J., Facchini P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008;59:735–769. - PubMed
-
- Nosov A.M. Application of cell technologies for production of plant-derived bioactive substances of plant origin. Appl. Biochem. Microbiol. 2012;48:609–624.
-
- Marco-Contelles J., do Carmo Carreiras M., Rodriguez C., Villarroya M., Garcia A.G. Synthesis and pharmacology of galanthamine. Chem. Rev. 2006;106:116–133. - PubMed
-
- De Luca V., Salim V., Atsumi S.M., Yu F. Mining the biodiversity of plants: A revolution in the making. Science. 2012;336:1658–1661. - PubMed
-
- Gates M., Tschudi G. The synthesis of morphine. J. Am. Chem. Soc. 1952;74:1109–1110.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

