Treatment failure and drug resistance is more frequent in HIV-1 subtype D versus subtype A-infected Ugandans over a 10-year study period
- PMID: 23727942
- PMCID: PMC4494684
- DOI: 10.1097/QAD.0b013e3283610ec7
Treatment failure and drug resistance is more frequent in HIV-1 subtype D versus subtype A-infected Ugandans over a 10-year study period
Abstract
Objectives: To determine the impact of HIV-1 subtype on treatment outcomes and the emergence of drug resistance in the resource limited setting of Kampala, Uganda.
Design: The Joint Clinical Research Centre (JCRC) in Kampala, Uganda has provided over 2000 drug-resistant genotypes (DRGs) over the past 10 years as standard of care for patients failing therapy and 1403 from treatment-naive and experienced patients over the past 10 years have been analyzed for this study.
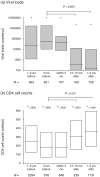
Method: Viral loads, CD4 cell count, treatment histories and other relevant clinical data was compared with the infecting HIV-1 subtype and DRGs of Ugandan patients failing treatment.
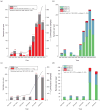
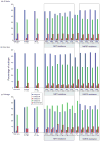
Results: Patients failing HAART with DRGs (n = 937) were more frequently infected with subtype D than expected on the basis of the subtype distribution in the treatment-naive population (n = 655) in Kampala (P < 0.001). Higher proportions of treatment failures among subtype D-infected patients were driven by resistance to nucleoside reverse transcriptase inhibitors (NRTI) (P < 0.0002) more than to non-NRTIs (P > 0.04) or protease inhibitors.
Conclusion: Higher rates of treatment failure among subtype D as compared with subtype A-infected Ugandans was analogous to the faster disease progression in subtype D-infected patients. The mechanism(s) by which drug resistance may emerge faster in subtype D HIV-1 may relate to higher replicative fitness and increased propensity for a CXCR4 tropism.
Conflict of interest statement
Figures

References
-
- Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbiize P, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–759. - PubMed
-
- Ojikutu B, Makadzange AT, Gaolathe T. Scaling up ART treatment capacity: lessons learned from South Africa, Zimbabwe, and Botswana. Curr Infect Dis Rep. 2008;10:69–73. - PubMed
-
- Taegtmeyer M, Chebet K. Overcoming challenges to the implementation of antiretroviral therapy in Kenya. Lancet Infect Dis. 2002;2:51–53. - PubMed
-
- Wood R, Lawn SD. Should the CD4 threshold for starting ART be raised? Lancet. 2009;373:1314–1316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

