Persistent G. lamblia impairs growth in a murine malnutrition model
- PMID: 23728173
- PMCID: PMC3668820
- DOI: 10.1172/JCI67294
Persistent G. lamblia impairs growth in a murine malnutrition model
Abstract
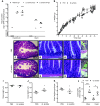
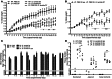
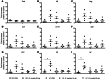
Giardia lamblia infections are nearly universal among children in low-income countries and are syndemic with the triumvirate of malnutrition, diarrhea, and developmental growth delays. Amidst the morass of early childhood enteropathogen exposures in these populations, G. lamblia–specific associations with persistent diarrhea, cognitive deficits, stunting, and nutrient deficiencies have demonstrated conflicting results, placing endemic pediatric giardiasis in a state of equipoise. Many infections in endemic settings appear to be asymptomatic/ subclinical, further contributing to uncertainty regarding a causal link between G. lamblia infection and developmental delay. We used G. lamblia H3 cyst infection in a weaned mouse model of malnutrition to demonstrate that persistent giardiasis leads to epithelial cell apoptosis and crypt hyperplasia. Infection was associated with a Th2-biased inflammatory response and impaired growth. Malnutrition accentuated the severity of these growth decrements. Faltering malnourished mice exhibited impaired compensatory responses following infection and demonstrated an absence of crypt hyperplasia and subsequently blunted villus architecture. Concomitantly, severe malnutrition prevented increases in B220+ cells in the lamina propria as well as mucosal Il4 and Il5 mRNA in response to infection. These findings add insight into the potential role of G. lamblia as a "stunting" pathogen and suggest that, similarly, malnourished children may be at increased risk of G. lamblia– potentiated growth decrements.
Figures



Comment in
-
Giardia: both a harmless commensal and a devastating pathogen.J Clin Invest. 2013 Jun;123(6):2352-4. doi: 10.1172/JCI69932. J Clin Invest. 2013. PMID: 23728170 Free PMC article.
-
Infection: Modelling persistent giardiasis and malnutrition.Nat Rev Gastroenterol Hepatol. 2013 Jul;10(7):382. doi: 10.1038/nrgastro.2013.100. Epub 2013 Jun 11. Nat Rev Gastroenterol Hepatol. 2013. PMID: 23752824 No abstract available.
References
-
- Hill DR, Nash TE. Principles and Practices of Infectious Diseases. 2009. Giardia lamblia. In: Mandell GL, Bennett JE, Dolin R, eds. pp. 3527–3534. 5th ed. Philadelphia, Pennsylvania, USA: Churchill Livngstone Elsevier;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

