Exenatide and the treatment of patients with Parkinson's disease
- PMID: 23728174
- PMCID: PMC3668846
- DOI: 10.1172/JCI68295
Exenatide and the treatment of patients with Parkinson's disease
Abstract
BACKGROUND. There is increasing interest in methods to more rapidly and cost-efficiently investigate drugs that are approved for clinical use in the treatment of another condition. Exenatide is a type 2 diabetes treatment that has been shown to have neuroprotective/neurorestorative properties in preclinical models of neurodegeneration. METHODS. As a proof of concept, using a single-blind trial design, we evaluated the progress of 45 patients with moderate Parkinson's disease (PD), randomly assigned to receive subcutaneous exenatide injection for 12 months or to act as controls. Their PD was compared after overnight withdrawal of conventional PD medication using blinded video assessment of the Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS), together with several nonmotor tests, at baseline, 6 months, and 12 months and after a further 2-month washout period (14 months). RESULTS. Exenatide was well tolerated, although weight loss was common and l-dopa dose failures occurred in a single patient. Single-blinded rating of the exenatide group suggested clinically relevant improvements in PD across motor and cognitive measures compared with the control group. Exenatide-treated patients had a mean improvement at 12 months on the MDS-UPDRS of 2.7 points, compared with mean decline of 2.2 points in control patients (P = 0.037). CONCLUSION. These results demonstrate a potential cost-efficient approach through which preliminary clinical data of possible biological effects are obtainable, prior to undertaking the major investment required for double-blind trials of a potential disease-modifying drug in PD.
Trial registration: Clinicaltrials.gov NCT01174810.
Funding: Cure Parkinson's Trust.
Figures

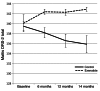
Comment in
-
A new approach to disease-modifying drug trials in Parkinson's disease.J Clin Invest. 2013 Jun;123(6):2364-5. doi: 10.1172/JCI69690. J Clin Invest. 2013. PMID: 23728166 Free PMC article.
References
-
- Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402–7405. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

