A spindle-independent cleavage pathway controls germ cell formation in Drosophila
- PMID: 23728423
- PMCID: PMC3818562
- DOI: 10.1038/ncb2761
A spindle-independent cleavage pathway controls germ cell formation in Drosophila
Abstract
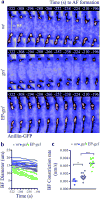
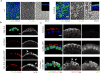
The primordial germ cells (PGCs) are the first cells to form during Drosophila melanogaster embryogenesis. Whereas the process of somatic cell formation has been studied in detail, the mechanics of PGC formation are poorly understood. Here, using four-dimensional multi-photon imaging combined with genetic and pharmacological manipulations, we find that PGC formation requires an anaphase spindle-independent cleavage pathway. In addition to using core regulators of cleavage, including the small GTPase RhoA (Drosophila rho1) and the Rho-associated kinase, ROCK (Drosophila drok), we show that this pathway requires Germ cell-less (GCL), a conserved BTB-domain protein not previously implicated in cleavage mechanics. This alternative form of cell formation suggests that organisms have evolved multiple molecular strategies for regulating the cytoskeleton during cleavage.
Figures



References
-
- Sullivan W, Theurkauf WE. The cytoskeleton and morphogenesis of the early Drosophila embryo. Current opinion in cell biology. 1995;7:18–22. - PubMed
-
- Glover DM. Mitosis in the Drosophila embryo--in and out of control. Trends Genet. 1991;7:125–132. - PubMed
-
- Edgar BA, Kiehle CP, Schubiger G. Cell cycle control by the nucleo-cytoplasmic ratio in early Drosophila development. Cell. 1986;44:365–372. - PubMed
-
- Foe VE, Field CM, Odell GM. Microtubules and mitotic cycle phase modulate spatiotemporal distributions of F-actin and myosin II in Drosophila syncytial blastoderm embryos. Development. 2000;127:1767–1787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

