Rivastigmine for vascular cognitive impairment
- PMID: 23728651
- PMCID: PMC11972847
- DOI: 10.1002/14651858.CD004744.pub3
Rivastigmine for vascular cognitive impairment
Abstract
Background: Vascular dementia represents the second most common type of dementia after Alzheimer's disease. In older patients, in particular, the combination of vascular dementia and Alzheimer's disease is common, and is referred to as mixed dementia. The classification of vascular dementia broadly follows three clinico-pathological processes: multi-infarct dementia, single strategic infarct dementia and subcortical dementia. Not all victims fulfil strict criteria for dementia and may be significantly cognitively impaired without memory loss, when the term vascular cognitive impairment (VCI) is more useful. Currently, no established standard treatment for VCI exists. Reductions in acetylcholine and acetyltransferase activity are common to both Alzheimer's disease and VCI, raising the possibility that cholinesterase inhibitors - such as rivastigmine - which are beneficial in Alzheimer's disease, may also be beneficial for VCI.
Objectives: To assess the efficacy of rivastigmine compared with placebo in the treatment of people with vascular cognitive impairment (VCI), vascular dementia or mixed dementia.
Search methods: We searched ALOIS (the Cochrane Dementia and Cognitive Improvement Group's Specialized Register) on 12 February 2013 using the terms: rivastigmine, exelon, "SDZ ENA 713". ALOIS contains records of clinical trials identified from monthly searches of a number of major healthcare databases (The Cochrane Library, MEDLINE, EMBASE, CINAHL, PsycINFO, LILACS), numerous trial registries and grey literature sources.
Selection criteria: All unconfounded randomized double-blind trials comparing rivastigmine with placebo in the treatment of people with VCI, vascular dementia or mixed dementia were eligible for inclusion.
Data collection and analysis: Two reviewers extracted and assessed data independently, and agreement was reached after discussion. They noted results concerning adverse effects.
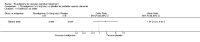
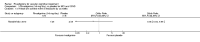
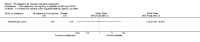
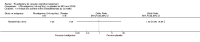
Main results: Three trials, with a total of 800 participants, were identified for inclusion. The participants in one trial did not have dementia, while the other two studies included participants with dementia of different severities. The dose of rivastigmine was different in each study. No pooling of study results was attempted because of these differences between the studies.One trial included 40 participants with subcortical vascular dementia (age range 40 to 90 years) with a mean mini-mental state examination (MMSE) score of 13.0 and 13.4 in the rivastigmine and placebo arms, respectively. Treatment over 26 weeks was limited to 3 mg rivastigmine twice daily, or placebo. No significant difference was found on any outcome measure relevant to cognition, neuropsychiatric symptoms, function or global rating, or in the number of withdrawals before the end of treatment.Another trial included 710 participants with vascular dementia, including subcortical and cortical forms (age range 50 to 85 years). Over 24 weeks, a mean dose of rivastigmine of 9.4 mg/day was achieved versus placebo. Baseline MMSE was identical for both groups, at 19.1. Statistically significant advantage in cognitive response (but not with global impression of change or non-cognitive measures) was seen with rivastigmine treatment at 24 weeks (MMSE change from baseline MD 0.6, 95% CI 0.11 to 1.09, P value 0.02; Vascular Dementia Assessment Scale (VaDAS) change from baseline MD -1.3, 95% CI-2.62 to 0.02, P value 0.05 ). Significantly higher rates of vomiting, nausea, diarrhoea and anorexia and withdrawals from treatment were noted in the participants randomized to rivastigmine compared with placebo (withdrawals rivastigmine 90/365, placebo 48/345, OR 2.02, 95% CI 1.38 to 2.98) (withdrawals due to an adverse event rivastigmine 49/365, placebo 19/345, OR 2.66, 95% CI 1.53 to 4.62, P value 0.0005).The third study included 50 participants (age range 48 to 84 years) with mean MMSE scores of 23.7 and 23.9 in the rivastigmine and placebo arms, respectively. Over a 24-week period, participants labelled as having cognitive impairment but no dementia (CIND) following ischaemic stroke were given up to 4.5 mg rivastigmine twice daily, or placebo. Primary and secondary outcome measures showed no statistically significant difference when considering neurocognitive abilities, function, neuropsychiatric symptoms and global performance. One participant in the rivastigmine group and two in the placebo group discontinued their medication because of an adverse effect.
Authors' conclusions: There is some evidence of benefit of rivastigmine in VCI from trial data from three studies. However, this conclusion is based on one large study. Rivastigmine is capable of inducing side effects that lead to withdrawal in a significant proportion of patients.
Conflict of interest statement
None known
Figures
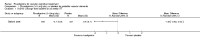


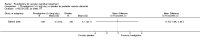
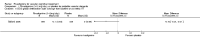
























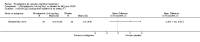









Update of
-
Rivastigmine for vascular cognitive impairment.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004744. doi: 10.1002/14651858.CD004744.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2013 May 31;(5):CD004744. doi: 10.1002/14651858.CD004744.pub3. PMID: 15846730 Updated.
References
References to studies included in this review
Ballard 2008 {published data only}
-
- Ballard C, Sauter M, Scheltens P, He Y, Barkhof F, Straaten EC, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Current medical research and opinion 2008;24(9):2561‐74. [PUBMED: 18674411] - PubMed
Mok 2007 {published data only}
Narasimhalu 2010 {published data only}
-
- Narasimhalu K, Effendy S, Sim CH, Lee JM, Chen I, Hia SB, et al. A randomized controlled trial of rivastigmine in patients with cognitive impairment no dementia because of cerebrovascular disease. Acta Neurologica Scandinavica 2010;121:217‐24. - PubMed
Additional references
Black 2011
-
- Black SE. Vascular cognitive impairment: epidemiology, subtypes, diagnosis and management. The journal of the Royal College of Physicians of Edinburgh 2011;41(1):49‐56. [PUBMED: 21365068] - PubMed
Bruandet 2009
-
- Bruandet A, Richard F, Bombois S, Maurage CA, Deramecourt V, Lebert F, et al. Alzheimer disease with cerebrovascular disease and vascular dementia: clinical features and course compared with Alzheimer disease. Journal of Neurology, Neurosurgery, and Psychiatry 2009;80(2):133‐9. [PUBMED: 18977819] - PubMed
Bullock 2004
-
- Bullock R. Cholinesterase inhibitors and vascular dementia: another string to their bow?. CNS Drugs 2004;18(2):79‐92. - PubMed
Chiu 1994
-
- Chiu HFK, Lee HC, Chung WS, Kwong PK. The reliability and validity of the Cantonese version of Mini‐Mental State Examination. East Asian Archives of Psychiatry: Official Journal of the Hong Kong College of Psychiatrists 1994;4 (Supp 2):25‐8.
Chui 1992
-
- Chui H, Victoroff J, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992;42(3 Part 1):473‐80. - PubMed
Cummings 1993
-
- Cummings JL. Frontal‐subcortical circuits and human behavior. Archives of Neurology 1993;50(8):873‐80. - PubMed
D'elia 1996
-
- D'elia LSP, Uchiyama CL, White T. Colour Trails Test. Psychological Assessment Resources Inc. PAR, 1996.
Dubois 2000
-
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000;55(11):1621‐6. [PUBMED: 11113214] - PubMed
Erkinjuntti 2000
-
- Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. Journal of Neural Transmission 2000;59(Suppl):23‐30. - PubMed
Ferris 1999
-
- Ferris SH. Cognitive outcome measures. Alzheimer Disease and Associated Disorders 1999;13 Suppl 3:S140‐2. [PUBMED: 10609693] - PubMed
Folstein 1975
-
- Folstein MF, Folstein SE, McHugh PR. 'Mini‐Mental State': A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. - PubMed
Galasko 1997
-
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:S33‐9. [PUBMED: 9236950] - PubMed
Hansen 2008
Higgins 2011
-
- Higgins JPT, Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
Kerola 2010
-
- Kerola T, Kettunen R, Nieminen T. The complex interplay of cardiovascular system and cognition: How to predict dementia in the elderly?. International Journal of Cardiology 2010;150(2):123‐9. [PUBMED: 21094551] - PubMed
Kivipelto 2005
-
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of Neurology 2005;62(10):1556‐60. [PUBMED: 16216938] - PubMed
Leung 2001
-
- Leung VP, Lam LC, Chiu HF, Cummings JL, Chen QL. Validation study of the Chinese version of the neuropsychiatric inventory (CNPI). International Journal of Geriatric Psychiatry 2001;16(8):789‐93. [PUBMED: 11536346] - PubMed
Malouf 2004
Manos 1994
-
- Manos PJ, Wu R. The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. International Journal of Psychiatry in Medicine 1994;24(3):229‐44. [PUBMED: 7890481] - PubMed
Mok 2004
-
- Mok VC, Wong A, Yim P, Fu M, Lam WW, Hui AC, et al. The validity and reliability of chinese frontal assessment battery in evaluating executive dysfunction among Chinese patients with small subcortical infarct. Alzheimer Disease and Associated Disorders 2004;18:68‐74. - PubMed
Moretti 2004
-
- Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Ukmar M, et al. Rivastigmine superior to aspirin plus nimodipine in subcortical vascular dementia: an open, 16‐month, comparative study. International Journal of Clinical Practice 2004;58(4):346‐53. - PubMed
Morris 1993
-
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43(11):2412‐4. [PUBMED: 8232972] - PubMed
MRC CFAS 2001
-
- Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of late‐onset dementia in a multicentre, community‐based population in England and Wales. The Lancet 2001;357:169‐75. - PubMed
O'Brien 2000
-
- O'Brien J, Perry R, Barber R, Gholkar A, Thomas A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Annals of the New York Academy of Sciences 2000;903:482‐9. - PubMed
O'Brien 2003
-
- O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. The Lancet Neurology 2003;2:89‐98. - PubMed
Perry 1997
-
- Perry E, Kay DW. Some developments in brain ageing and dementia. British Journal of Biomedical Science 1997;54(3):201‐15. - PubMed
Pohjasvaara 2003
-
- Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Clinical features of MRI‐defined subcortical vascular disease. Alzheimer Disease and Associated Disorders 2003;17(4):236‐42. - PubMed
Reisberg 1982
-
- Reisberg B, Ferris SH, Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. The American Journal of Psychiatry 1982;139(9):1136‐9. [PUBMED: 7114305] - PubMed
Roman 1993
-
- Roman G, Tatemichi T, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. - PubMed
Rosen 1984
-
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. The American Journal of Psychiatry 1984;141(11):1356‐64. [PUBMED: 6496779] - PubMed
Schneider 1997
-
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:S22‐32. [PUBMED: 9236949] - PubMed
Toghi 1996
-
- Toghi H, Abe T, Kimura M, Saheki M, Takahashi S. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarcts types as compared with Alzheimer‐type dementia. Journal of Neural Transmission 1996;103:1211‐20. - PubMed
Tong 2002
-
- Tong AYC, Man DWK. The Validation of the Hong Kong Chinese Version of the Lawton Instrumental Activities of Daily Living Scale for institutionalized elderly persons.. Occupation, Participation and Health 2002;22(4):132.
WHO 1992
-
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Description and Diagnostic Guidelines. 10th Edition. Geneva: World Health organiazation, 1992.
Yesavage 1982
-
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 1982;17(1):37‐49. [PUBMED: 7183759] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

