Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus
- PMID: 23728671
- PMCID: PMC11822331
- DOI: 10.1002/14651858.CD008107.pub2
Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus
Abstract
Background: The outcome of patients with locally advanced gastroesophageal adenocarcinoma (adenocarcinoma of the esophagus, gastroesophageal (GE) junction, and stomach) is poor. There is conflicting evidence regarding the effects of perioperative chemotherapy on survival and other outcomes.
Objectives: To assess the effect of perioperative chemotherapy for gastroesophageal adenocarcinoma on survival and other clinically relevant outcomes in the overall population of participants in randomized controlled trials (RCTs) and in prespecified subgroups.
Search methods: We performed computerized searches in the Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Review of Effectiveness (DARE), the Cochrane Database of Systematic Reviews (CDSR) from The Cochrane Library, MEDLINE (1966 to May 2011), EMBASE (1980 to May 2011), and LILACS (Literatura Latinoamericana y del Caribe en Ciencias de la Salud), combining the Cochrane highly sensitive search strategy with specific search terms. Moreover, we handsearched several online databases, conference proceedings, and reference lists of retrieved papers.
Selection criteria: We included RCTs which randomized patients with gastroesophageal adenocarcinoma, in the absence of distant metastases, to receive either chemotherapy with or without radiotherapy followed by surgery, or surgery alone.
Data collection and analysis: Two independent review authors identified eligible trials. We solicited individual patient data (IPD) from all selected trials. We performed meta-analyses based on intention-to-treat populations using the two-stage method to combine IPD with aggregate data from RCTs for which IPD were unavailable. We combined data from all trials providing IPD in a Cox proportional hazards model to assess the effect of several covariables on overall survival.
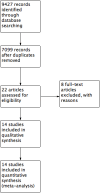
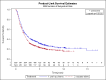
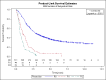
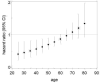
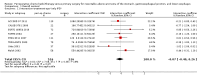
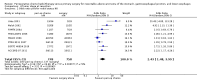
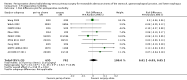
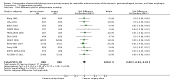
Main results: We identified 14 RCTs with 2422 eligible patients. For eight RCTs with 1049 patients (43.3%), we were able to obtain IPD. Perioperative chemotherapy was associated with significantly longer overall survival (hazard ratio (HR) 0.81; 95% confidence interval (CI) 0.73 to 0.89). This corresponds to a relative survival increase of 19% or an absolute survival increase of 9% at five years. This survival advantage was consistent across most subgroups. There was a trend towards a more pronounced treatment effect for tumors of the GE junction compared to other sites, and for combined chemoradiotherapy as compared to chemotherapy in tumors of the esophagus and GE junction. Resection with negative margins was a strong predictor of survival. Multivariable analysis showed that tumor site, performance status, and age have an independent significant effect on survival. Moreover, there was a significant interaction of the effect of perioperative chemotherapy with age (larger treatment effect in younger patients). Perioperative chemotherapy also showed a significant effect on several secondary outcomes. It was associated with longer disease-free survival, higher rates of R0 resection, and more favorable tumor stage upon resection, while there was no association with perioperative morbidity and mortality.
Authors' conclusions: Perioperative chemotherapy for resectable gastroesophageal adenocarcinoma increases survival compared to surgery alone. It should thus be offered to all eligible patients. There is a trend to a larger survival advantage for tumors of the GE junction as compared to other sites and for chemoradiotherapy as compared to chemotherapy in esophageal and GE junction tumors. Likewise, there is an interaction between age and treatment effect, with younger patients having a larger survival advantage, and no survival advantage for elderly patients.
Conflict of interest statement
RH has participated in several trials assessing the effect of various chemotherapeutic agents on upper GI tract cancer.
Figures






















Update of
References
References to studies included in this review
ACCORD 07 2011 {published data only}
-
- Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. Journal of Clinical Oncology 2011;29(13):1715‐21. - PubMed
CALGB 9781 2008 {published data only}
EORTC 40954 2010 {published data only}
-
- Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. Journal of Clinical Oncology 2010;28(35):5210‐8. - PMC - PubMed
FAMTX 2004 {published data only}
-
- Hartgrink HH, van de Velde CJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK, et al. Neo‐adjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. European Journal of Surgical Oncology 2004;30(6):643‐9. - PubMed
Feng 2008 {published data only}
-
- Feng L‐M, Li G, Sun X‐C, Zhu S‐G. Treatment out come of neoadjuvant chemotherapy for Bormann's type IV gastric cancer. Chinese Journal of Cancer Prevention and Treatment 2008;15(13):1022‐4.
Kobayashi 2000 {published data only}
-
- Kobayashi T. Long‐term outcome of preoperative chemotherapy with 5'‐deoxy‐5‐fluorouridine (5'‐DFUR) for gastric cancer. Gan to Kagaku Ryoho 2000;27(10):1521‐6. - PubMed
MAGIC 2006 {published data only}
-
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New England Journal of Medicine 2006;355(1):11‐20. - PubMed
OE02 2009 {published data only}
-
- Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. Journal of Clinical Oncology 2009;27(30):5062‐7. - PubMed
RTOG 8911 2007 {published data only}
-
- Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, et al. Long‐term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. Journal of Clinical Oncology 2007;25(24):3719‐25. - PubMed
TROG‐AGITG 2005 {published data only}
-
- Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncology 2005;6(9):659‐68. - PubMed
Urba 2001 {published data only}
-
- Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. Journal of Clinical Oncology 2001;19(2):305‐13. - PubMed
Walsh 2002 {published data only}
-
- Walsh TN, Grennell M, Mansoor S, Kelly A. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Diseases of the Esophagus 2002;15(2):121‐4. - PubMed
Wang 2000 {published data only}
-
- Wang XL, Wu GX, Zhang MD, Guo M, Zhang H, Sun XF. A favorable impact of preoperative FPLC chemotherapy on patients with gastric cardia cancer. Oncology Reports 2000;7(2):241‐4. - PubMed
References to studies excluded from this review
Bokhyan 2009 {published data only}
-
- Bokhyan V, Stilidi I, Malikhova O, Tryakin A. Neoadjuvant chemotherapy followed by transthoracic resection for locally advanced carcinoma of the esophagus: A randomized study. European Journal of Cancer, Supplement. Elsevier Ltd, 2009; Vol. 7, issue 2‐3:377.
Furlong 2010 {published data only}
-
- Furlong H, Gilani N, Atie M, Bass G, Mulligan E, Keeling N, et al. Is the short‐term survival advantage for neoadjuvant chemoradiotherapy in oesophageal cancer sustained? Long‐term follow‐up of two randomised trials. Diseases of the Esophagus. Blackwell Publishing, 2010; Vol. 23:74A.
Jin 2008 {published data only}
-
- Jin M‐G, Jiang S‐C, Chen Z‐W, Wang Z‐Q. Clinical trial of preoperative concurrent chemoradiation followed by surgery versus surgery alone for advanced esophageal carcinoma. Chinese Journal of Cancer Prevention and Treatment 2008;15(23):1815‐7.
Jin 2011 {published data only}
-
- Jin FL, Hu ZL, Ma HF. Treatment effect of neoadjuvant chemoradiotherapy followed by surgery versus surgery alone in local advanced esophageal carcinoma. Journal of Practical Oncology. Journal of Practical Oncology, Editorial Board (31009China), 2011; Vol. 26, issue 5:523‐6.
Mariette 2010 {published data only}
-
- Mariette C, Seitz JF, Maillard E, Mornex F, Thomas PA, Raoul J, et al. Surgery alone versus chemoradiotherapy followed by surgery for localized esophageal cancer: Analysis of a randomized controlled phase III trial FFCD 9901. ASCO Meeting Abstracts 2010;28(15 Suppl):4005.
Pokataev 2008 {published data only}
-
- Pokataev I, Tryakin A, Stilidi I, Kononets P, Polotskiy B, Malikhova O, et al. Preoperative chemotherapy followed by surgery versus surgery alone in resectable esophageal cancer: A single institute phase III TRIAL. Annals of Oncology. Oxford University Press, 2008; Vol. 19, issue S8:viii170.
van Hagen 2012 {published data only}
-
- Hagen P, Hulshof MCCM, Lanschot JJB, Steyerberg EW, Henegouwen MI, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New England Journal of Medicine 2012;366(22):2074‐84. - PubMed
Additional references
Ajani 1993
-
- Ajani JA, Mayer RJ, Ota DM, Steele GD, Evans D, Roh M, et al. Preoperative and postoperative combination chemotherapy for potentially resectable gastric carcinoma. Journal of the National Cancer Institute 1993;85(22):1839‐44. - PubMed
Ajani 1995
-
- Ajani JA, Mansfield PF, Ota DM. Potentially resectable gastric carcinoma: current approaches to staging and preoperative therapy. World Journal of Surgery 1995;19(2):216‐20. - PubMed
Ajani 2004
-
- Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, et al. Multi‐institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. Journal of Clinical Oncology 2004;22(14):2774‐80. - PubMed
Ajani 2005
-
- Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, et al. Paclitaxel‐based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. Journal of Clinical Oncology 2005;23(6):1237‐44. - PubMed
Ajani 2006
-
- Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. Journal of Clinical Oncology 2006;24(24):3953‐8. - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. - PubMed
Biffi 2010
Burmeister 2011
-
- Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. European Journal of Cancer 2011;47(3):354‐60. - PubMed
Chau 2004
-
- Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago‐gastric cancer‐‐pooled analysis from three multicenter, randomized, controlled trials using individual patient data. Journal of Clinical Oncology 2004;22(12):2395‐403. [PUBMED: 15197201] - PubMed
Chau 2009
-
- Chau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T, et al. The impact of primary tumour origins in patients with advanced oesophageal, oesophago‐gastric junction and gastric adenocarcinoma‐‐individual patient data from 1775 patients in four randomised controlled trials. Annals of Oncology 2009;20(5):885‐91. - PubMed
DeMeester 2006
-
- DeMeester SR. Adenocarcinoma of the esophagus and cardia: a review of the disease and its treatment. Annals of Surgical Oncology 2006;13(1):12‐30. - PubMed
Dikken 2011
Djarv 2010
-
- Djarv T, Metcalfe C, Avery KN, Lagergren P, Blazeby JM. Prognostic value of changes in health‐related quality of life scores during curative treatment for esophagogastric cancer. Journal of Clinical Oncology 2010;28(10):1666‐70. [PUBMED: 20194863] - PubMed
Djarv 2012
-
- Djarv T, Lagergren P. Quality of life after esophagectomy for cancer. Expert Review of Gastroenterology and Hepatology 2012;6(1):115‐22. [PUBMED: 22149587] - PubMed
Egger 1997
Eguchi 2008
-
- Eguchi T, Kodera Y, Nakanishi H, Yokoyama H, Ohashi N, Ito Y, et al. The effect of chemotherapy against micrometastases and isolated tumor cells in lymph nodes: an in vivo study. In Vivo 2008;22(6):707‐12. [PUBMED: 19180995] - PubMed
Ferlay 2010
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. - PubMed
Fisher 2011
-
- Fisher DJ, Copas AJ, Tierney JF, Parmar MKB. A critical review of methods for the assessment of patient‐level interactions in individual participant data meta‐analysis of randomized trials, and guidance for practitioners. Journal of Clinical Epidemiology 2011;64:949‐67. - PubMed
Forman 2006
-
- Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Practice and Research. Clinical Gastroenterology 2006;20(4):633‐49. - PubMed
Gallo 2006
Hagen 2001
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kelsen 1996
-
- Kelsen DP. Adjuvant and neoadjuvant therapy for gastric cancer. Seminars in Oncology 1996;23(3):379‐89. - PubMed
Macdonald 2001
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New England Journal of Medicine 2001;345(10):725‐30. - PubMed
Malthaner 2004
Marsman 2005
-
- Marsman WA, Tytgat GN, Kate FJ, Lanschot JJ. Differences and similarities of adenocarcinomas of the esophagus and esophagogastric junction. Journal of Surgical Oncology 2005;92(3):160‐8. - PubMed
McCulloch 2003
Munene 2012
-
- Munene G, Francis W, Garland SN, Pelletier G, Mack LA, Bathe OF. The quality of life trajectory of resected gastric cancer. Journal of Surgical Oncology 2012;105(4):337‐41. [PUBMED: 22095440] - PubMed
Ott 2003
-
- Ott K, Sendler A, Becker K, Dittler HJ, Helmberger H, Busch R, et al. Neoadjuvant chemotherapy with cisplatin, 5‐FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer 2003;6(3):159‐67. - PubMed
Paoletti 2010
-
- Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta‐analysis. JAMA 2010;303(17):1729‐37. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Peters 2006
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;295:676–80. - PubMed
Pignon 2001
-
- Pignon JP, Hill C. Meta‐analyses of randomised clinical trials in oncology. Lancet 2001;2:475‐82. - PubMed
R 2010 [Computer program]
-
- R Foundation for Statistical Computing. R. Version 2.11.0. Vienna: R Foundation for Statistical Computing, 2010.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Riley 2007
-
- Riley RD, Simmonds MC, Look MP. Evidence synthesis combining individual patient data and aggregate data: a systematic review identified current practice and possible methods. Journal of Clinical Epidemiology 2007;60:431‐9. - PubMed
Riley 2010
-
- Riley RD, Lambert PC, Abo‐Zaid G. Meta‐analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. - PubMed
SAS 2011 [Computer program]
-
- SAS Institute. SAS. Version 9.2 and 9.3. Cary, North Carolina: SAS Institute, 2008 and 2011.
Schemper 1996
-
- Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Controlled Clinical Trials 1996;17:343‐6. - PubMed
Shah 2011
Siewert 1987
-
- Siewert JR, Holscher AH, Becker K, Gossner W. Cardia cancer: attempt at a therapeutically relevant classification [Kardiacarcinom: Versuch einer therapeutisch relevanten Klassifikation]. Chirurg 1987;58(1):25‐32. - PubMed
Siewert 1998
Sjoquist 2011
-
- Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. Lancet Oncology 2011;7:681‐92. - PubMed
Slanger 2009
-
- Slanger TE, Schwarzbach M, Hofheinz R, Kienle P, Jensen K, Kieser M, et al. Perioperative chemotherapy versus primary surgery for locoregionally advanced resectable adenocarcinoma of the stomach, gastroesophageal junction and lower esophagus. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008107] - DOI - PMC - PubMed
Sobin 2002
-
- Sobin LH, Wittekind C, Sobin LH, Wittekind C. In: Sobin LH, Wittekind C editor(s). TNM Classification of Malignant Tumours (UICC). New York: Wiley‐Liss, 2002.
Stahl 2009
-
- Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera‐Knorrenschild J, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. Journal of Clinical Oncology 2009;27(6):851‐6. - PubMed
Stewart 1995
-
- Stewart LA, Clarke MJ. Practical methodology of meta‐analyses (overviews) using updated individual patient data. Statistics in Medicine 1995;14:2057‐79. - PubMed
Thirion 2007
-
- Thirion PG, Michiels S, Maître A, Tierney J, on behalf of the MetaAnalysis of Chemotherapy in Esophagus Cancer Collaborative Group. Individual patient data‐based meta‐analysis assessing pre‐operative chemotherapy in resectable oesophageal carcinoma. Journal of Clinical Oncology 2007;25(18S):4512.
Thirion 2008
-
- Thirion P, Maillard E, Pignon J. Individual patient data‐based meta‐analysis assessing the effect of preoperative chemo‐radiotherapy in resectable oesophageal carcinoma. International Journal of Radiation Oncology, Biology, Physics. Elsevier Science Inc., 2008; Vol. 72, issue 1:S71‐2.
Tierney 2007
Verger 1992
-
- Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group Scoring Scale and vice‐versa. European Journal of Cancer 1992;28A(8‐9):1328‐30. - PubMed
Vial 2010
-
- Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results in Cancer Research 2010;182:1‐17. - PubMed
Vogt 2006
Williamson 2002
-
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

