Extracellular electron transfer to Fe(III) oxides by the hyperthermophilic archaeon Geoglobus ahangari via a direct contact mechanism
- PMID: 23728807
- PMCID: PMC3719510
- DOI: 10.1128/AEM.01566-13
Extracellular electron transfer to Fe(III) oxides by the hyperthermophilic archaeon Geoglobus ahangari via a direct contact mechanism
Abstract
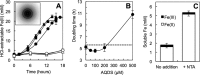
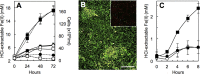
The microbial reduction of Fe(III) plays an important role in the geochemistry of hydrothermal systems, yet it is poorly understood at the mechanistic level. Here we show that the obligate Fe(III)-reducing archaeon Geoglobus ahangari uses a direct-contact mechanism for the reduction of Fe(III) oxides to magnetite at 85°C. Alleviating the need to directly contact the mineral with the addition of a chelator or the electron shuttle anthraquinone-2,6-disulfonate (AQDS) stimulated Fe(III) reduction. In contrast, entrapment of the oxides within alginate beads to prevent cell contact with the electron acceptor prevented Fe(III) reduction and cell growth unless AQDS was provided. Furthermore, filtered culture supernatant fluids had no effect on Fe(III) reduction, ruling out the secretion of an endogenous mediator too large to permeate the alginate beads. Consistent with a direct contact mechanism, electron micrographs showed cells in intimate association with the Fe(III) mineral particles, which once dissolved revealed abundant curled appendages. The cells also produced several heme-containing proteins. Some of them were detected among proteins sheared from the cell's outer surface and were required for the reduction of insoluble Fe(III) oxides but not for the reduction of the soluble electron acceptor Fe(III) citrate. The results thus support a mechanism in which the cells directly attach and transfer electrons to the Fe(III) oxides using redox-active proteins exposed on the cell surface. This strategy confers on G. ahangari a competitive advantage for accessing and reducing Fe(III) oxides under the extreme physical and chemical conditions of hot ecosystems.
Figures


References
-
- Martin W, Baross J, Kelley D, Russell MJ. 2008. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6:805–814 - PubMed
-
- Karl DM. 1995. Ecology of free-living hydrothermal vent microbial communities, p 35–124 In Karl DM. (ed), The microbiology of deep-sea hydrothermal vents. CRC Press, New York, NY
-
- Vargas M, Kashefi K, Blunt-Harris EL, Lovley DR. 1998. Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65–67 - PubMed
-
- Kashefi K. 2012. Hyperthermophiles: metabolic diversity and biotechnological applications, p 183–231 In Antinori RP. (ed), Extremophiles: microbiology and biotechnology. Caister Academic Press, Norfolk, United Kingdom
-
- Heimann A, Johnson CM, Beard BL, Valley JW, Roden EE, Spicuzza MJ, Beukes NJ. 2010. Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in similar to 2.5 Ga marine environments. Earth Planet. Sci. Lett. 294:8–18
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

