Mechanobiology in lung epithelial cells: measurements, perturbations, and responses
- PMID: 23728969
- PMCID: PMC4457445
- DOI: 10.1002/cphy.c100090
Mechanobiology in lung epithelial cells: measurements, perturbations, and responses
Abstract
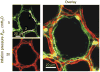
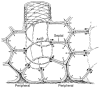
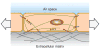
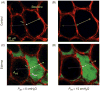
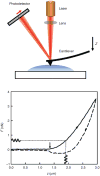
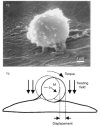
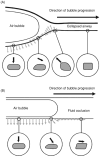
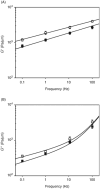
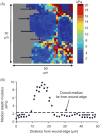
Epithelial cells of the lung are located at the interface between the environment and the organism and serve many important functions including barrier protection, fluid balance, clearance of particulate, initiation of immune responses, mucus and surfactant production, and repair following injury. Because of the complex structure of the lung and its cyclic deformation during the respiratory cycle, epithelial cells are exposed to continuously varying levels of mechanical stresses. While normal lung function is maintained under these conditions, changes in mechanical stresses can have profound effects on the function of epithelial cells and therefore the function of the organ. In this review, we will describe the types of stresses and strains in the lungs, how these are transmitted, and how these may vary in human disease or animal models. Many approaches have been developed to better understand how cells sense and respond to mechanical stresses, and we will discuss these approaches and how they have been used to study lung epithelial cells in culture. Understanding how cells sense and respond to changes in mechanical stresses will contribute to our understanding of the role of lung epithelial cells during normal function and development and how their function may change in diseases such as acute lung injury, asthma, emphysema, and fibrosis.
© 2012 American Physiological Society
Figures
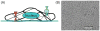







References
-
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–1308. - PubMed
-
- Adkins WK, Hernandez LA, Coker PJ, Buchanan B, Parker JC. Age effects susceptibility to pulmonary barotrauma in rabbits. Crit Care Med. 1991;19:390–393. - PubMed
-
- Agostoni E, Hyatt RE. Static behaviour of the respiratory system. In: Macklem PT, Mead J, editors. Handbook of Physiology Mechanics of Breathing. Bethesda, MD: American Physiological Society; 1986. pp. 113–130. sect. 3, pt. 1.
-
- Alcaraz J, Buscemi L, Puig-de-Morales M, Colchero J, Baro A, Navajas D. Correction of microrheological measurements of soft samples with atomic force microscopy for the hydrodynamic drag on the cantilever. Langmuir. 2002;18:716–721.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

