Peripheral chemoreceptors: function and plasticity of the carotid body
- PMID: 23728973
- PMCID: PMC3919066
- DOI: 10.1002/cphy.c100069
Peripheral chemoreceptors: function and plasticity of the carotid body
Abstract
The discovery of the sensory nature of the carotid body dates back to the beginning of the 20th century. Following these seminal discoveries, research into carotid body mechanisms moved forward progressively through the 20th century, with many descriptions of the ultrastructure of the organ and stimulus-response measurements at the level of the whole organ. The later part of 20th century witnessed the first descriptions of the cellular responses and electrophysiology of isolated and cultured type I and type II cells, and there now exist a number of testable hypotheses of chemotransduction. The goal of this article is to provide a comprehensive review of current concepts on sensory transduction and transmission of the hypoxic stimulus at the carotid body with an emphasis on integrating cellular mechanisms with the whole organ responses and highlighting the gaps or discrepancies in our knowledge. It is increasingly evident that in addition to hypoxia, the carotid body responds to a wide variety of blood-borne stimuli, including reduced glucose and immune-related cytokines and we therefore also consider the evidence for a polymodal function of the carotid body and its implications. It is clear that the sensory function of the carotid body exhibits considerable plasticity in response to the chronic perturbations in environmental O2 that is associated with many physiological and pathological conditions. The mechanisms and consequences of carotid body plasticity in health and disease are discussed in the final sections of this article.
© 2012 American Physiological Society
Figures
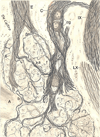












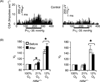



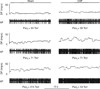
References
-
- Abbott CP, De Burgh Daly M, Howe A. Early ultrastructural changes in the carotid body after degenerative section of the carotid sinus nerve in the cat. Acta Anat. 1972;83:161–183. - PubMed
-
- Abraham A. Electron microscopic investigations on the human carotid body. (Preliminary communication) Z Mikrosk Anat Forsch. 1968;79:309–315. - PubMed
-
- Abu-Soud HM, Rousseau DL, Stuehr DJ. Nitric oxide binding to the heme of neuronal nitric-oxide synthase links its activity to changes in oxygen tension. J Biol Chem. 1996;271:32515–32518. - PubMed
-
- Abudara V, Garces G, Saez JC. Cells of the carotid body express connexin43 which is up-regulated by cAMP. Brain Res. 1999;849:25–33. - PubMed
-
- Abudara V, Jiang RG, Eyzaguirre C. Acidic regulation of junction channels between glomus cells in the rat carotid body. Possible role of [Ca(2+)](i) Brain Res. 2001;916:50–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

