Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer
- PMID: 23729478
- PMCID: PMC4078408
- DOI: 10.1158/2159-8290.CD-12-0558
Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer
Abstract
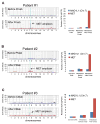
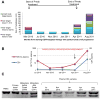
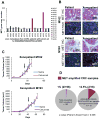
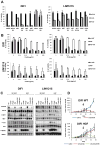
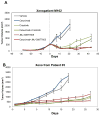
EGF receptor (EGFR)-targeted monoclonal antibodies are effective in a subset of metastatic colorectal cancers. Inevitably, all patients develop resistance, which occurs through emergence of KRAS mutations in approximately 50% of the cases. We show that amplification of the MET proto-oncogene is associated with acquired resistance in tumors that do not develop KRAS mutations during anti-EGFR therapy. Amplification of the MET locus was present in circulating tumor DNA before relapse was clinically evident. Functional studies show that MET activation confers resistance to anti-EGFR therapy both in vitro and in vivo. Notably, in patient-derived colorectal cancer xenografts, MET amplification correlated with resistance to EGFR blockade, which could be overcome by MET kinase inhibitors. These results highlight the role of MET in mediating primary and secondary resistance to anti-EGFR therapies in colorectal cancer and encourage the use of MET inhibitors in patients displaying resistance as a result of MET amplification.
Conflict of interest statement
T. Perera is a full-time employee of Janssen Pharmaceutica and a shareholder of Johnson and Johnson, the manufacturer of JNJ38877605. L.A.D. and V.E.V. are co-founders of Inostics and Personal Genome Diagnostics and are members of their Scientific Advisory Boards. L.A.D. and V.E.V. own Inostics and Personal Genome Diagnostics stock, which is subject to certain restrictions under University policy. The terms of these arrangements are managed by the Johns Hopkins University in accordance with its conflict-of-interest policies.
Figures

References
-
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74. - PubMed
-
- Rothenberg ML, LaFleur B, Levy DE, Washington MK, Morgan-Meadows SL, Ramanathan RK, et al. Randomized phase II trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. J Clin Oncol. 2005;23:9265–74. - PubMed
-
- Santoro A, Comandone A, Rimassa L, Granetti C, Lorusso V, Oliva C, et al. A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer. Ann Oncol. 2008;19:1888–93. - PubMed
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

