Perivascular macrophage-like melanocyte responsiveness to acoustic trauma--a salient feature of strial barrier associated hearing loss
- PMID: 23729595
- PMCID: PMC3752533
- DOI: 10.1096/fj.13-232892
Perivascular macrophage-like melanocyte responsiveness to acoustic trauma--a salient feature of strial barrier associated hearing loss
Abstract
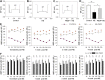
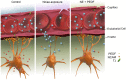
Tissue perivascular resident macrophages (PVM/Ms), a hybrid cell type with characteristics of both macrophages and melanocytes, are critical for establishing and maintaining the endocochlear potential (EP) required for hearing. The PVM/Ms modulate expression of tight- and adherens-junction proteins in the endothelial barrier of the stria vascularis (intrastrial fluid-blood barrier) through secretion of a signaling molecule, pigment epithelium growth factor (PEDF). Here, we identify a significant link between abnormalities in PVM/Ms and endothelial barrier breakdown from acoustic trauma to the mouse ear. We find that acoustic trauma causes activation of PVM/Ms and physical detachment from capillary walls. Concurrent with the detachment, we find loosened tight junctions between endothelial cells and decreased production of tight- and adherens-junction protein, resulting in leakage of serum proteins from the damaged barrier. A key factor in the intrastrial fluid-blood barrier hyperpermeability exhibited in the mice is down-regulation of PVM/M modulated PEDF production. We demonstrate that delivery of PEDF to the damaged ear ameliorates hearing loss by restoring intrastrial fluid-blood barrier integrity. PEDF up-regulates expression of tight junction-associated proteins (ZO-1 and VE-cadherin) and PVM/M stabilizing neural cell adhesion molecule (NCAM-120). These studies point to the critical role PVM/Ms play in regulating intrastrial fluid-blood barrier integrity in healthy and noise-damaged ears.
Keywords: acoustic trauma; endothelial cell; instrastrial fluid-blood barrier; mouse cochlea; paracellular permeability.
Figures




References
-
- Juhn S. K., Hunter B. A., Odland R. M. (2001) Blood-labyrinth barrier and fluid dynamics of the inner ear. Int. Tinnitus J. 7, 72–83 - PubMed
-
- Juhn S. K., Rybak L. P. (1981) Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol. 91, 529–534 - PubMed
-
- Lin D. W., Trune D. R. (1997) Breakdown of stria vascularis blood-labyrinth barrier in C3H/lpr autoimmune disease mice. Otolaryngol. Head Neck Surg. 117, 530–534 - PubMed
-
- Doi K., Mori N., Matsunaga T. (1992) Adenylate cyclase modulation of ion permeability in the guinea pig cochlea: a possible mechanism for the formation of endolymphatic hydrops. Acta Otolaryngol. 112, 667–673 - PubMed
-
- McMenomey S. O., Russell N. J., Morton J. I., Trune D. R. (1992) Stria vascularis ultrastructural pathology in the C3H/lpr autoimmune strain mouse: a potential mechanism for immune-related hearing loss. Otolaryngol. Head Neck Surg. 106, 288–295 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

