Inducible interleukin 32 (IL-32) exerts extensive antiviral function via selective stimulation of interferon λ1 (IFN-λ1)
- PMID: 23729669
- PMCID: PMC3774363
- DOI: 10.1074/jbc.M112.440115
Inducible interleukin 32 (IL-32) exerts extensive antiviral function via selective stimulation of interferon λ1 (IFN-λ1)
Abstract
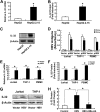
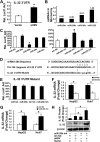
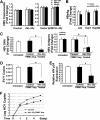
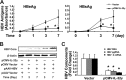
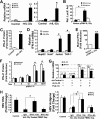
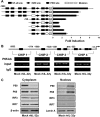
Interleukin (IL)-32 has been recognized as a proinflammatory cytokine that participates in responses to viral infection. However, little is known about how IL-32 is induced in response to viral infection and the mechanisms of IL-32-mediated antiviral activities. We discovered that IL-32 is elevated by hepatitis B virus (HBV) infection both in vitro and in vivo and that HBV induced IL-32 expression at the level of both transcription and post-transcription. Furthermore, microRNA-29b was found to be a key factor in HBV-regulated IL-32 expression by directly targeting the mRNA 3'-untranslated region of IL-32. Antiviral analysis showed that IL-32 was not sufficient to alter HBV replication in HepG2.2.15 cells. To mimic the viremic phase of viral infection, freshly isolated peripheral blood mononuclear cells were treated with IL-32γ, the secretory isoform, and the supernatants were used for antiviral assays. Surprisingly, these supernatants exhibited extensive antiviral activity against multiplex viruses besides HBV. Thus, we speculated that the IL-32γ-treated peripheral blood mononuclear cells produced and secreted an unknown antiviral factor. Using antibody neutralization assays, we identified the factor as interferon (IFN)-λ1 and not IFN-α. Further studies indicated that IL-32γ effectively inhibited HBV replication in a hydrodynamic injection mouse model. Clinical data showed that elevated levels of IFN-λ1 both in serum and liver tissue of HBV patients were positively correlated to the increased levels of IL-32. Our results demonstrate that elevated IL-32 levels during viral infection mediate antiviral effects by stimulating the expression of IFN-λ1.
Keywords: Cytokines/Interferon; Gene Regulation; Host-Pathogen Interactions; Infectious Diseases; Interleukin; Viral Immunology.
Figures



Similar articles
-
IL-27, a cytokine, and IFN-λ1, a type III IFN, are coordinated to regulate virus replication through type I IFN.J Immunol. 2014 Jan 15;192(2):691-703. doi: 10.4049/jimmunol.1300252. Epub 2013 Dec 11. J Immunol. 2014. PMID: 24337382
-
MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-λ1.Protein Cell. 2013 Feb;4(2):130-41. doi: 10.1007/s13238-012-2081-y. Epub 2012 Nov 12. Protein Cell. 2013. PMID: 23150165 Free PMC article.
-
Poly(I:C) treatment leads to interferon-dependent clearance of hepatitis B virus in a hydrodynamic injection mouse model.J Virol. 2014 Sep;88(18):10421-31. doi: 10.1128/JVI.00996-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920792 Free PMC article.
-
Increased interleukin-32, interleukin-1, and interferon-γ levels in serum from hepatitis B patients and in HBV-stimulated peripheral blood mononuclear cells from healthy volunteers.J Infect Public Health. 2019 Jan-Feb;12(1):7-12. doi: 10.1016/j.jiph.2018.06.006. Epub 2018 Jul 10. J Infect Public Health. 2019. PMID: 30006119 Review.
-
Contribution of Toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses.Cell Mol Immunol. 2015 May;12(3):273-82. doi: 10.1038/cmi.2014.112. Epub 2014 Nov 24. Cell Mol Immunol. 2015. PMID: 25418467 Free PMC article. Review.
Cited by
-
MicroRNA-29b-3p suppresses oral squamous cell carcinoma cell migration and invasion via IL32/AKT signalling pathway.J Cell Mol Med. 2020 Jan;24(1):841-849. doi: 10.1111/jcmm.14794. Epub 2019 Nov 3. J Cell Mol Med. 2020. PMID: 31680452 Free PMC article.
-
Allele-specific long-distance regulation dictates IL-32 isoform switching and mediates susceptibility to HIV-1.Sci Adv. 2018 Feb 21;4(2):e1701729. doi: 10.1126/sciadv.1701729. eCollection 2018 Feb. Sci Adv. 2018. PMID: 29507875 Free PMC article.
-
Lower Plasma IL-32 Levels Linked to Better Survival in Sepsis.Biomedicines. 2025 Mar 19;13(3):750. doi: 10.3390/biomedicines13030750. Biomedicines. 2025. PMID: 40149726 Free PMC article.
-
Gene Expression and Antiviral Activity of Interleukin-35 in Response to Influenza A Virus Infection.J Biol Chem. 2016 Aug 5;291(32):16863-76. doi: 10.1074/jbc.M115.693101. Epub 2016 Jun 15. J Biol Chem. 2016. PMID: 27307042 Free PMC article.
-
IL-33 facilitates proliferation of colorectal cancer dependent on COX2/PGE2.J Exp Clin Cancer Res. 2018 Aug 17;37(1):196. doi: 10.1186/s13046-018-0839-7. J Exp Clin Cancer Res. 2018. PMID: 30119635 Free PMC article.
References
-
- Netea M. G., Azam T., Lewis E. C., Joosten L. A., Wang M., Langenberg D., Meng X., Chan E. D., Yoon D.-Y., Ottenhoff T., Kim S.-H., Dinarello C. A. (2006) Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-γ-dependent mechanism. PLoS Med. 3, e277. - PMC - PubMed
-
- Nold M. F., Nold-Petry C. A., Pott G. B., Zepp J. A., Saavedra M. T., Kim S. H., Dinarello C. A. (2008) Endogenous IL-32 controls cytokine and HIV-1 production. J. Immunol. 181, 557–565 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

