CDC25A protein stability represents a previously unrecognized target of HER2 signaling in human breast cancer: implication for a potential clinical relevance in trastuzumab treatment
- PMID: 23730206
- PMCID: PMC3665943
- DOI: 10.1593/neo.122054
CDC25A protein stability represents a previously unrecognized target of HER2 signaling in human breast cancer: implication for a potential clinical relevance in trastuzumab treatment
Abstract
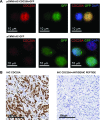
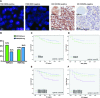
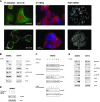
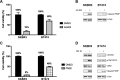
The CDC25A-CDK2 pathway has been proposed as critical for the oncogenic action of human epidermal growth factor receptor 2 (HER2) in mammary epithelial cells. In particular, transgenic expression of CDC25A cooperates with HER2 in promoting mammary tumors, whereas CDC25A hemizygous loss attenuates the HER2-induced tumorigenesis penetrance. On the basis of this evidence of a synergism between HER2 and the cell cycle regulator CDC25A in a mouse model of mammary tumorigenesis, we investigated the role of CDC25A in human HER2-positive breast cancer and its possible implications in therapeutic response. HER2 status and CDC25A expression were assessed in 313 breast cancer patients and we found statistically significant correlation between HER2 and CDC25A (P = .007). Moreover, an HER2-positive breast cancer subgroup with high levels of CDC25A and very aggressive phenotype was identified (P = .005). Importantly, our in vitro studies on breast cancer cell lines showed that the HER2 inhibitor efficacy on cell growth and viability relied also on CDC25A expression and that such inhibition induces CDC25A down-regulation through phosphatidylinositol 3-kinase/protein kinase B pathway and DNA damage response activation. In line with this observation, we found a statistical significant association between CDC25A overexpression and trastuzumab-combined therapy response rate in two different HER2-positive cohorts of trastuzumab-treated patients in either metastatic or neoadjuvant setting (P = .018 for the metastatic cohort and P = .021 for the neoadjuvant cohort). Our findings highlight a link between HER2 and CDC25A that positively modulates HER2-targeted therapy response, suggesting that, in HER2-positive breast cancer patients, CDC25A overexpression affects trastuzumab sensitivity.
Figures


References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. - PubMed
-
- Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, Schlessinger J, Lippman ME, King CR. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
