High-throughput screening identifies aclacinomycin as a radiosensitizer of EGFR-mutant non-small cell lung cancer
- PMID: 23730419
- PMCID: PMC3660808
- DOI: 10.1593/tlo.13232
High-throughput screening identifies aclacinomycin as a radiosensitizer of EGFR-mutant non-small cell lung cancer
Abstract
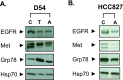
The endoplasmic reticulum (ER) provides a specialized environment for the folding and modification of trans-membrane proteins, including receptor tyrosine kinases (RTKs), which are vital for the growth and survival of malignancies. To identify compounds which disrupt the function of the ER and thus could potentially impair cancer cell survival signaling, we adapted a set of glycosylation-sensitive luciferase reporters for the development and optimization of a cell-based high-throughput screen (HTS). Secondary screens for false-positive luciferase activation and tertiary lectin-based and biochemical analyses were also devised for compound triage. Through a pilot screen of 2802 compounds from the National Cancer Institute (NCI) chemical libraries, we identified aclacinomycin (Acm) as a compound that preferentially affects ER function. We report that Acm reduces plasma membrane expression of glycoproteins including epidermal growth factor receptor (EGFR) and Met but does not inhibit N-linked glycosylation or generalized protein translation. Fluorescence microscopy co-localization experiments were also performed and demonstrated Acm accumulation in the ER in further support of the overall HTS design. The consequences of Acm treatment on cell survival were analyzed through clonogenic survival analysis. Consistent with the reduction of EGFR levels, pretreatment with Acm sensitizes the EGFR-mutant non-small cell lung cancer (NSCLC) cell lines HCC827 and HCC2935 to ionizing radiation and did not affect the sensitivity of the RTK-independent and KRAS-mutant A549 NSCLC cell line. Thus, Acm and similar compounds targeting the ER may represent a novel approach for radiosensitizing tumor cells dependent on RTK function.
Figures




References
-
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. - PubMed
-
- Laisney JA, Mueller TD, Schartl M, Meierjohann S. Hyper-activation of constitutively dimerized oncogenic EGF receptors by autocrine loops. Oncogene. 2012 E-pub ahead of print. - PubMed
-
- Wu YM, Liu CH, Hu RH, Huang MJ, Lee JJ, Chen CH, Huang J, Lai HS, Lee PH, Hsu WM, et al. Mucin glycosylating enzyme GALNT2 regulates the malignant character of hepatocellular carcinoma by modifying the EGF receptor. Cancer Res. 2011;71:7270–7279. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
