Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models
- PMID: 23730620
- PMCID: PMC3656359
- DOI: 10.3389/fonc.2013.00097
Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models
Abstract
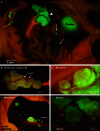
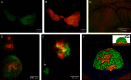
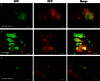
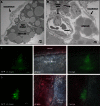
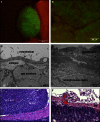
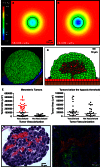
Ovarian cancer relapse is often characterized by metastatic spread throughout the peritoneal cavity with tumors attached to multiple organs. In this study, interaction of ovarian cancer cells with the peritoneal tumor microenvironment was evaluated in a xenograft model based on intraperitoneal injection of fluorescent SKOV3.ip1 ovarian cancer cells. Intra-vital microscopy of mixed GFP-red fluorescent protein (RFP) cell populations injected into the peritoneum demonstrated that cancer cells aggregate and attach as mixed spheroids, emphasizing the importance of homotypic adhesion in tumor formation. Electron microscopy provided high resolution structural information about local attachment sites. Experimental measurements from the mouse model were used to build a three-dimensional cellular Potts ovarian tumor model (OvTM) that examines ovarian cancer cell attachment, chemotaxis, growth, and vascularization. OvTM simulations provide insight into the relative influence of cancer cell-cell adhesion, oxygen availability, and local architecture on tumor growth and morphology. Notably, tumors on the mesentery, omentum, or spleen readily invade the "open" architecture, while tumors attached to the gut encounter barriers that restrict invasion and instead rapidly expand into the peritoneal space. Simulations suggest that rapid neovascularization of SKOV3.ip1 tumors is triggered by constitutive release of angiogenic factors in the absence of hypoxia. This research highlights the importance of cellular adhesion and tumor microenvironment in the seeding of secondary ovarian tumors on diverse organs within the peritoneal cavity. Results of the OvTM simulations indicate that invasion is strongly influenced by features underlying the mesothelial lining at different sites, but is also affected by local production of chemotactic factors. The integrated in vivo mouse model and computer simulations provide a unique platform for evaluating targeted therapies for ovarian cancer relapse.
Keywords: angiogenesis; cell adhesion; cellular Potts model; chemotaxis; metastasis; ovarian cancer; tumor microenvironment; tumor modeling.
Figures

Similar articles
-
In vitro mesothelial clearance assay that models the early steps of ovarian cancer metastasis.J Vis Exp. 2012 Feb 17;(60):3888. doi: 10.3791/3888. J Vis Exp. 2012. PMID: 22371143 Free PMC article.
-
Omentum-on-a-chip: A multicellular, vascularized microfluidic model of the human peritoneum for the study of ovarian cancer metastases.Biomaterials. 2022 Sep;288:121728. doi: 10.1016/j.biomaterials.2022.121728. Epub 2022 Aug 16. Biomaterials. 2022. PMID: 35995621
-
Modeling the Early Steps of Ovarian Cancer Dissemination in an Organotypic Culture of the Human Peritoneal Cavity.J Vis Exp. 2015 Dec 31;(106):e53541. doi: 10.3791/53541. J Vis Exp. 2015. PMID: 26780294 Free PMC article.
-
Intraperitoneal metastasis of ovarian cancer: new insights on resident macrophages in the peritoneal cavity.Front Immunol. 2023 Apr 25;14:1104694. doi: 10.3389/fimmu.2023.1104694. eCollection 2023. Front Immunol. 2023. PMID: 37180125 Free PMC article. Review.
-
The Mesothelial Origin of Carcinoma Associated-Fibroblasts in Peritoneal Metastasis.Cancers (Basel). 2015 Sep 29;7(4):1994-2011. doi: 10.3390/cancers7040872. Cancers (Basel). 2015. PMID: 26426054 Free PMC article. Review.
Cited by
-
Rheological transition driven by matrix makes cancer spheroids resilient under confinement.Life Sci Alliance. 2025 Mar 27;8(6):e202402601. doi: 10.26508/lsa.202402601. Print 2025 Jun. Life Sci Alliance. 2025. PMID: 40147946 Free PMC article.
-
LYVE1+ macrophages of murine peritoneal mesothelium promote omentum-independent ovarian tumor growth.J Exp Med. 2021 Dec 6;218(12):e20210924. doi: 10.1084/jem.20210924. Epub 2021 Oct 29. J Exp Med. 2021. PMID: 34714329 Free PMC article.
-
Lattice-based model of ductal carcinoma in situ suggests rules for breast cancer progression to an invasive state.PLoS Comput Biol. 2014 Dec 4;10(12):e1003997. doi: 10.1371/journal.pcbi.1003997. eCollection 2014 Dec. PLoS Comput Biol. 2014. PMID: 25473842 Free PMC article.
-
Immune Tumor Microenvironment in Ovarian Cancer Ascites.Int J Mol Sci. 2022 Sep 14;23(18):10692. doi: 10.3390/ijms231810692. Int J Mol Sci. 2022. PMID: 36142615 Free PMC article. Review.
-
Ovarian Tumor Microenvironment Signaling: Convergence on the Rac1 GTPase.Cancers (Basel). 2018 Sep 27;10(10):358. doi: 10.3390/cancers10100358. Cancers (Basel). 2018. PMID: 30261690 Free PMC article. Review.
References
-
- Bagley R. G., Walter-Yohrling J., Cao X., Weber W., Simons B., Cook B. P., et al. (2003). Endothelial precursor cells as a model of tumor endothelium. Cancer Res. 63, 5866–5873 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

