New Strategies to Direct Therapeutic Targeting of PML to Treat Cancers
- PMID: 23730625
- PMCID: PMC3656422
- DOI: 10.3389/fonc.2013.00124
New Strategies to Direct Therapeutic Targeting of PML to Treat Cancers
Abstract
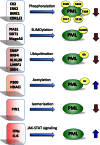
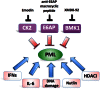
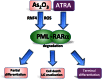
The tumor suppressor function of the promyelocytic leukemia (PML) protein was first identified as a result of its dysregulation in acute promyelocytic leukemia, however, its importance is now emerging far beyond hematological neoplasms, to an extensive range of malignancies, including solid tumors. In response to stress signals, PML coordinates the regulation of numerous proteins, which activate fundamental cellular processes that suppress tumorigenesis. Importantly, PML itself is the subject of specific post-translational modifications, including ubiquitination, phosphorylation, acetylation, and SUMOylation, which in turn control PML activity and stability and ultimately dictate cellular fate. Improved understanding of the regulation of this key tumor suppressor is uncovering potential opportunities for therapeutic intervention. Targeting the key negative regulators of PML in cancer cells such as casein kinase 2, big MAP kinase 1, and E6-associated protein, with specific inhibitors that are becoming available, provides unique and exciting avenues for restoring tumor suppression through the induction of apoptosis and senescence. These approaches could be combined with DNA damaging drugs and cytokines that are known to activate PML. Depending on the cellular context, reactivation or enhancement of tumor suppressive PML functions, or targeted elimination of aberrantly functioning PML, may provide clinical benefit.
Keywords: BMK1; CK2; E6AP; KLHL20; PML; small molecule inhibitors; targeted anti-cancer therapy; tumour suppression.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

