Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization
- PMID: 23730946
- PMCID: PMC3870485
- DOI: 10.1089/ten.TEC.2012.0731
Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization
Abstract
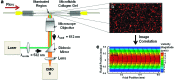
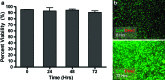
Hyperpermeable tumor vessels are responsible for elevated interstitial fluid pressure and altered flow patterns within the tumor microenvironment. These aberrant hydrodynamic stresses may enhance tumor development by stimulating the angiogenic activity of endothelial cells lining the tumor vasculature. However, it is currently not known to what extent shear forces affect endothelial organization or paracrine signaling during tumor angiogenesis. The objective of this study was to develop a three-dimensional (3D), in vitro microfluidic tumor vascular model for coculture of tumor and endothelial cells under varying flow shear stress conditions. A central microchannel embedded within a collagen hydrogel functions as a single neovessel through which tumor-relevant hydrodynamic stresses are introduced and quantified using microparticle image velocimetry (μ-PIV). This is the first use of μ-PIV in a tumor representative, 3D collagen matrix comprised of cylindrical microchannels, rather than planar geometries, to experimentally measure flow velocity and shear stress. Results demonstrate that endothelial cells develop a confluent endothelium on the microchannel lumen that maintains integrity under physiological flow shear stresses. Furthermore, this system provides downstream molecular analysis capability, as demonstrated by quantitative RT-PCR, in which, tumor cells significantly increase expression of proangiogenic genes in response to coculture with endothelial cells under low flow conditions. This work demonstrates that the microfluidic in vitro cell culture model can withstand a range of physiological flow rates and permit quantitative measurement of wall shear stress at the fluid-collagen interface using μ-PIV optical flow diagnostics, ultimately serving as a versatile platform for elucidating the role of fluid forces on tumor-endothelial cross talk.
Figures






References
-
- Butler T.P., Grantham F.H., and Gullino P.M.Bulk transfer of fluid in the interstitial compartment of mammary tumors. Cancer Res 35,3084, 1975 - PubMed
-
- Vaupel P., Kallinowski F., and Okunieff P.Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49,6449, 1989 - PubMed
-
- Fukumura D., and Jain R.K.Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem 101,937, 2007 - PubMed
-
- Jain R.K.Determinants of tumor blood flow: a review. Cancer Res 48,2641, 1988 - PubMed
-
- Jain R.K.Vascular and Interstitial Biology of Tumors. Philadelphia: Elsevier, 2004
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

