Transitional B cell subsets in human bone marrow
- PMID: 23731328
- PMCID: PMC3784213
- DOI: 10.1111/cei.12149
Transitional B cell subsets in human bone marrow
Abstract
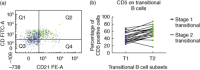
B cells originate from precursors in the bone marrow, and the first cells which migrate to the peripheral blood have been classified as 'transitional B cells'. Transitional B cells have been characterized in human blood with stage 1 (T1) and stage 2 (T2) subsets being proposed. In the present study, 27 normal human bone marrow samples were analysed for transitional B cell markers by eight-colour flow cytometry. T1 transitional B cells (CD45(+)CD19(+)CD10(+)IgM(+)IgD(lo)) and T2 transitional B cells (CD45(+)CD19(+)CD10(+)IgM(+)IgD(+)) were identified in normal bone marrow samples at a mean frequency of 3·2 and 3·1% of total B lineage cells, respectively. A majority of the bone marrow transitional B cells were CD24(hi)CD38(hi) , the phenotype of blood transitional B cells. Consistent with recent peripheral blood data, T2 B cells had a significantly higher CD21 expression compared with T1 B cells (72·4 versus 40·9%) in the bone marrow. These data raise the possibility that transitional B cells are capable of differentiating from T1 to T2 B cells within the bone marrow. Furthermore, transitional cells at either stages 1 or 2 might be capable of migrating out of the bone marrow.
Keywords: CD21; bone marrow; flow cytometry; human; transitional B cells.
© 2013 British Society for Immunology.
Figures




References
-
- Hoebeke I, De Smedt M, Stolz F, et al. T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(–)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia. 2007;21:311–319. - PubMed
-
- Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. - PubMed
-
- Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

