pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test
- PMID: 23731660
- PMCID: PMC3686627
- DOI: 10.1186/1475-2875-12-181
pLDH level of clinically isolated Plasmodium vivax and detection limit of pLDH based malaria rapid diagnostic test
Abstract
Background: The malaria rapid diagnostic tests (RDTs) are now widely used in the world. Compared to Plasmodium falciparum, a poor sensitivity of RDTs was reported against Plasmodium vivax based on the adopted antibody against pan-Plasmodium antigen lactate dehydrogenase (pLDH) or aldolase. Levels of pLDH were measured from patient with P. vivax, and the correlations between the levels of pLDH and the sensitivities of RDTs were analysed among Republic of Korea (ROK) isolates.
Methods: Three RDTs, OptiMAL test, SD BIOLINE Malaria Ag P.f/Pan test, Humasis Malaria Pf/Pan antigen test, and the Genedia pLDH antigen ELISA were performed with blood samples from 152 febrile patients and 100 healthy controls.
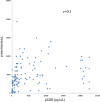
Results: Three malaria RDTs revealed sensitivities between 85.5 (131/152) and 86.8% (132/152) with highest sensitivity for the detection of P.vivax by pLDH antigen ELISA test (145/152, 95.4%) in comparison to traditional microscopy using Giemsa-stained slides. None of the healthy control tested positive by three RDTs or ELISA, indicating 100% specificity in their respective test. Levels of pLDH among Korean P. vivax isolates ranged between 0 ng/mL and 22,387.2 ng/mL (mean ± standard deviation 3,917.5 ± 6,120.9 ng/mL). The lower detection limits of three RDTs were between 25 and 50 ng/mL with artificially diluted samples. The moderate degree of correlation was observed between parasitaemia and concentrations of pLDH (r = 0.4, p < 0.05).
Conclusion: The pLDH levels of P. vivax are the main explanation for the variations in the performance of pLDH-based RDTs. Therefore, comparing sensitivities of RDT may need to include targeted biomarker value of patients.
Figures
References
-
- Makler MT, Hindrich DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources