Quinazoline derivatives: synthesis and bioactivities
- PMID: 23731671
- PMCID: PMC3679743
- DOI: 10.1186/1752-153X-7-95
Quinazoline derivatives: synthesis and bioactivities
Abstract
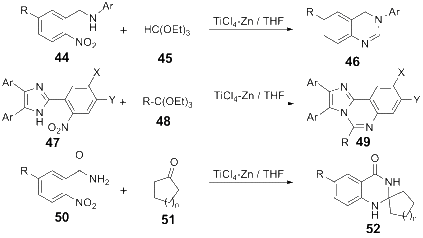
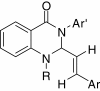
Owing to the significant biological activities, quinazoline derivatives have drawn more and more attention in the synthesis and bioactivities research. This review summarizes the recent advances in the synthesis and biological activities investigations of quinazoline derivatives. According to the main method the authors adopted in their research design, those synthetic methods were divided into five main classifications, including Aza-reaction, Microwave-assisted reaction, Metal-mediated reaction, Ultrasound-promoted reaction and Phase-transfer catalysis reaction. The biological activities of the synthesized quinazoline derivatives also are discussed.
Figures


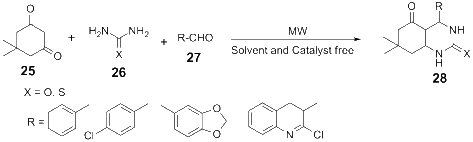
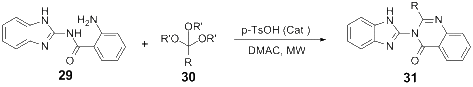
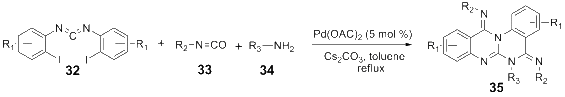

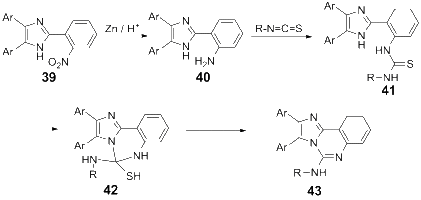
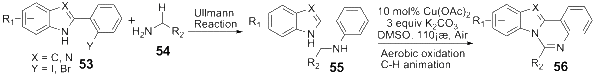
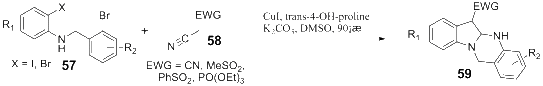
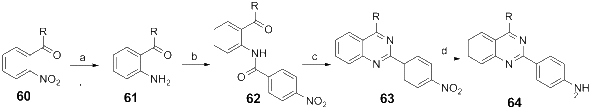
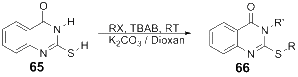
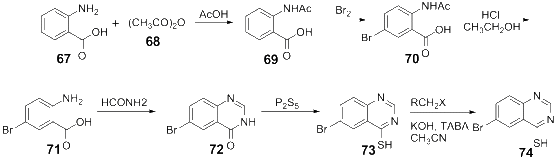

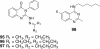
References
-
- Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AA, Abdel-Hamide SG, Youssef KM, Al-Obaid AM, El-Subbagh HI. Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem. 2006;14:8608–8621. doi: 10.1016/j.bmc.2006.08.030. - DOI - PubMed
-
- Vasdev N, Dorff PN, Gibbs AR, Nandanan E, Reid LM, Neil JPO’, VanBrocklin HF. Synthesis of 6-acrylamido-4-(2-[18F] fluoroanilino) quinazoline: A prospective irreversible EGFR binding probe. J Lablelled Compd Rad. 2005;48:109–115. doi: 10.1002/jlcr.903. - DOI
-
- Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

