Persistence, immune response, and antigenic variation of Mycoplasma genitalium in an experimentally infected pig-tailed macaque (Macaca nemestrina)
- PMID: 23732170
- PMCID: PMC3719596
- DOI: 10.1128/IAI.01322-12
Persistence, immune response, and antigenic variation of Mycoplasma genitalium in an experimentally infected pig-tailed macaque (Macaca nemestrina)
Abstract
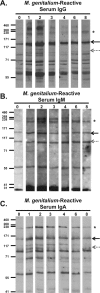
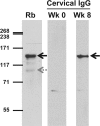
Mycoplasma genitalium is a sexually transmitted pathogen associated with several acute and chronic reproductive tract disease syndromes in men and women. To evaluate the suitability of a pig-tailed macaque model of M. genitalium infection, we inoculated a pilot animal with M. genitalium strain G37 in the uterine cervix and in salpingeal pockets generated by transplanting autologous Fallopian tube tissue subcutaneously. Viable organisms were recovered throughout the 8-week experiment in cervicovaginal specimens and up to 2 weeks postinfection in salpingeal pockets. Humoral and cervicovaginal antibodies reacting to MgpB were induced postinoculation and persisted throughout the infection. The immunodominance of the MgpB adhesin and the accumulation of mgpB sequence diversity previously observed in persistent human infections prompted us to evaluate sequence variation in this animal model. We found that after 8 weeks of infection, sequences within mgpB variable region B were replaced by novel sequences generated by reciprocal recombination with an archived variant sequence located elsewhere on the chromosome. In contrast, mgpB region B of the same inoculum propagated for 8 weeks in vitro remained unchanged. Notably, serum IgG reacted strongly with a recombinant protein spanning MgpB region B of the inoculum, while reactivity to a recombinant protein representing the week 8 variant was reduced, suggesting that antibodies were involved in the clearance of bacteria expressing the original infecting sequence. Together these results suggest that the pig-tailed macaque is a suitable model to study M. genitalium pathogenesis, antibody-mediated selection of antigenic variants in vivo, and immune escape.
Figures



Similar articles
-
The Unique Microbiology and Molecular Pathogenesis of Mycoplasma genitalium.J Infect Dis. 2017 Jul 15;216(suppl_2):S382-S388. doi: 10.1093/infdis/jix172. J Infect Dis. 2017. PMID: 28838077 Free PMC article. Review.
-
Analysis of the Mycoplasma genitalium MgpB Adhesin to Predict Membrane Topology, Investigate Antibody Accessibility, Characterize Amino Acid Diversity, and Identify Functional and Immunogenic Epitopes.PLoS One. 2015 Sep 18;10(9):e0138244. doi: 10.1371/journal.pone.0138244. eCollection 2015. PLoS One. 2015. PMID: 26381903 Free PMC article.
-
Experimental Infection of Pig-Tailed Macaques (Macaca nemestrina) with Mycoplasma genitalium.Infect Immun. 2017 Jan 26;85(2):e00738-16. doi: 10.1128/IAI.00738-16. Print 2017 Feb. Infect Immun. 2017. PMID: 27872239 Free PMC article.
-
Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences.Infect Immun. 2006 Jul;74(7):3715-26. doi: 10.1128/IAI.00239-06. Infect Immun. 2006. PMID: 16790744 Free PMC article.
-
Pathogenicity and virulence of Mycoplasma genitalium: Unraveling Ariadne's Thread.Virulence. 2022 Dec;13(1):1161-1183. doi: 10.1080/21505594.2022.2095741. Virulence. 2022. PMID: 35791283 Free PMC article. Review.
Cited by
-
Sequence variation and immunogenicity of the Mycoplasma genitalium MgpB and MgpC adherence proteins during persistent infection of men with non-gonococcal urethritis.PLoS One. 2020 Oct 12;15(10):e0240626. doi: 10.1371/journal.pone.0240626. eCollection 2020. PLoS One. 2020. PMID: 33045031 Free PMC article.
-
Mycoplasma genitalium adhesin P110 binds sialic-acid human receptors.Nat Commun. 2018 Oct 26;9(1):4471. doi: 10.1038/s41467-018-06963-y. Nat Commun. 2018. PMID: 30367053 Free PMC article.
-
The Unique Microbiology and Molecular Pathogenesis of Mycoplasma genitalium.J Infect Dis. 2017 Jul 15;216(suppl_2):S382-S388. doi: 10.1093/infdis/jix172. J Infect Dis. 2017. PMID: 28838077 Free PMC article. Review.
-
Activation of σ20-dependent recombination and horizontal gene transfer in Mycoplasma genitalium.DNA Res. 2018 Aug 1;25(4):383-393. doi: 10.1093/dnares/dsy011. DNA Res. 2018. PMID: 29659762 Free PMC article.
-
Analysis of the Mycoplasma genitalium MgpB Adhesin to Predict Membrane Topology, Investigate Antibody Accessibility, Characterize Amino Acid Diversity, and Identify Functional and Immunogenic Epitopes.PLoS One. 2015 Sep 18;10(9):e0138244. doi: 10.1371/journal.pone.0138244. eCollection 2015. PLoS One. 2015. PMID: 26381903 Free PMC article.
References
-
- Horner PJ, Gilroy CB, Thomas BJ, Naidoo RO, Taylor-Robinson D. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582–585 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

