Regulation of dopamine D₃ receptor in the striatal regions and substantia nigra in diffuse Lewy body disease
- PMID: 23732230
- PMCID: PMC3796121
- DOI: 10.1016/j.neuroscience.2013.05.048
Regulation of dopamine D₃ receptor in the striatal regions and substantia nigra in diffuse Lewy body disease
Abstract
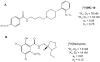
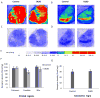
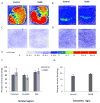
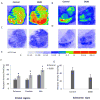
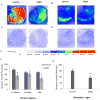
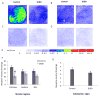
The regulation of D₃ receptor has not been well documented in diffuse Lewy body disease (DLBD). In this study, a novel D₃-preferring radioligand [(3)H]WC-10 and a D₂-preferring radioligand [(3)H]raclopride were used and the absolute densities of the dopamine D₃ and D₂ receptors were determined in the striatal regions and substantia nigra (SN) from postmortem brains from five cases of DLBD, which included dementia with Lewy bodies (DLB, n=4) and Parkinson disease dementia (PDD, n=1). The densities of the dopamine D₁ receptor, vesicular monoamine transporter 2 (VMAT2), and dopamine transporter (DAT) were also measured by quantitative autoradiography using [(3)H]SCH23390, [(3)H]dihydrotetrabenazine, and [(3)H]WIN35428, respectively. The densities of these dopaminergic markers were also measured in the same brain regions in 10 age-matched control cases. Dopamine D₃ receptor density was significantly increased in the striatal regions including caudate, putamen and nucleus accumbens (NAc). There were no significant changes in the dopamine D₁ and D₂ receptor densities in any brain regions measured. VMAT2 and DAT densities were reduced in all the brain regions measured in DLB/PDD, however, the significant reduction was found in the putamen for DAT and in the NAc and SN for VMAT2. The decrease of dopamine pre-synaptic markers implies neuronal loss in the substantia nigra pars compacta (SNpc) in these DLB/PDD cases, while the increase of D₃ receptors in striatal regions could be attributed to dopaminergic medication history and psychiatric states such as hallucinations. Whether it also reflects compensatory regulation upon dopaminergic denervation warrants further confirmations on larger populations.
Keywords: Parkinson disease; diffuse Lewy body disease; dopamine receptors; quantitative autoradiography.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures


References
-
- Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: a study with positron emission tomography and [11C]raclopride. Mov Disord. 1997;12:33–38. - PubMed
-
- Antonini A, Vontobel P, Psylla M, Gunther I, Maguire PR, Missimer J, Leenders KL. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s disease. Archives of neurology. 1995;52:1183–1190. - PubMed
-
- Barone P, Bankiewicz KS, Corsini GU, Kopin IJ, Chase TN. Dopaminergic mechanisms in hemiparkinsonian monkeys. Neurology. 1987a;37:1592–1595. - PubMed
-
- Barone P, Tucci I, Parashos SA, Chase TN. D-1 dopamine receptor changes after striatal quinolinic acid lesion. Eur J Pharmacol. 1987b;138:141–145. - PubMed
-
- Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 2003;9:762–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG05681/AG/NIA NIH HHS/United States
- R21 MH081281/MH/NIMH NIH HHS/United States
- NS075321/NS/NINDS NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- R01 NS075321/NS/NINDS NIH HHS/United States
- R21 DA016181/DA/NIDA NIH HHS/United States
- R03 NS090214/NS/NINDS NIH HHS/United States
- R33 MH081281/MH/NIMH NIH HHS/United States
- DA29840/DA/NIDA NIH HHS/United States
- R01 NS058714/NS/NINDS NIH HHS/United States
- RF1 NS075321/NS/NINDS NIH HHS/United States
- MH081281/MH/NIMH NIH HHS/United States
- R01 DA029840/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

