Mechanisms of mitochondrial fission and fusion
- PMID: 23732471
- PMCID: PMC3660830
- DOI: 10.1101/cshperspect.a011072
Mechanisms of mitochondrial fission and fusion
Abstract
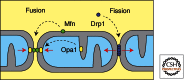
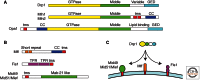
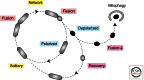
Mitochondria continually change shape through the combined actions of fission, fusion, and movement along cytoskeletal tracks. The lengths of mitochondria and the degree to which they form closed networks are determined by the balance between fission and fusion rates. These rates are influenced by metabolic and pathogenic conditions inside mitochondria and by their cellular environment. Fission and fusion are important for growth, for mitochondrial redistribution, and for maintenance of a healthy mitochondrial network. In addition, mitochondrial fission and fusion play prominent roles in disease-related processes such as apoptosis and mitophagy. Three members of the Dynamin family are key components of the fission and fusion machineries. Their functions are controlled by different sets of adaptor proteins on the surface of mitochondria and by a range of regulatory processes. Here, we review what is known about these proteins and the processes that regulate their actions.
Figures

References
-
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, et al. 2000. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215 - PubMed
-
- Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C 2005. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem 280: 35742–35750 - PubMed
-
- Baricault L, Segui B, Guegand L, Olichon A, Valette A, Larminat F, Lenaers G 2007. OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res 313: 3800–3808 - PubMed
-
- Bereiter-Hahn J, Voth M 1994. Dynamics of mitochondria in living cells: Shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech 27: 198–219 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
