Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial
- PMID: 23732903
- PMCID: PMC3906258
- DOI: 10.4161/hv.24846
Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial
Abstract
Rotavirus is the most common cause of severe gastroenteritis in children under 5 y of age. Estimates of disease burden in Japan suggest that between 26,500 and 78,000 children in this age group need hospitalization each year, resulting in a direct medical cost of 10 to 24 billion Yen. Since being introduced in routine infant immunization schedules in the United States in 2006, the oral live pentavalent rotavirus vaccine RV5 (RotaTeq™) has contributed to dramatic reductions in the incidence of rotavirus gastroenteritis (RVGE) and in health care resource utilization. This was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of a 3-dose regimen of RV5 in healthy infants, age 6 to 12 weeks, at 32 sites across Japan. The results indicate that RV5 was significantly efficacious in preventing any severity [74.5% (95% confidence interval [CI]: 39.9%, 90.6%; p<0.001)], moderate-to-severe [80.2% (95% CI: 47.4%, 94.1%)], and severe [100% (95% CI: 55.4%, 100%)] RVGE caused by viruses with serotypes contained in the vaccine. The observed cases of RVGE included rotavirus types G1 (n=19), G3 (n=9), G9 (n=5) and one unspecified G serotype with P1A[8]. No G2 or G4 RVGE cases were observed, and this study was not powered to evaluate efficacy against individual serotypes. RV5 was generally safe and well tolerated in Japanese infants. These results are comparable to those observed in clinical studies conducted in other developed countries. Introduction of the vaccine in Japan may reduce disease burden and associated health care costs.
Keywords: RV5; RVGE; pentavalent rotavirus vaccine; rotavirus gastroenteritis; vaccine.
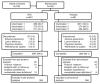
Figures
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
