Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand
- PMID: 23732904
- PMCID: PMC3906265
- DOI: 10.4161/hv.24844
Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand
Abstract
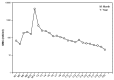
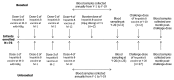
Hepatitis B vaccine has been available worldwide since the mid-1980s. This vaccine was evaluated in a clinical trial in Thailand, conducted on subjects born to hepatitis B surface antigen positive and hepatitis B e-antigen positive mothers and vaccinated according to a 4-dose schedule at 0, 1, 2 and 12 mo of age and a single dose of hepatitis B immunoglobulin concomitantly at birth. All enrolled subjects seroconverted and were followed for 20 y to assess the persistence of antibody to the hepatitis B surface antigen (anti-HBs) (NCT00240539). At year 20, 64% of subjects had anti-HBs antibody concentrations≥10 milli-international units per milli liter (mIU/ml) and 92% of subjects had detectable levels (≥3.3 mIU/ml) of anti-HBs antibodies. At year 20, subjects with anti-HBs antibody titer<100 mIU/ml were offered an additional dose of hepatitis B virus (HBV) vaccine to assess immune memory (NCT00657657). Anamnestic response to the challenge dose was observed in 96.6% of subjects with an 82-fold (13.2 to 1082.4 mIU/ml) increase in anti-HBs antibody geometric mean concentrations. This study confirms the long-term immunogenicity of the 4-dose regimen of the HBV vaccine eliciting long-term persistence of antibodies and immune memory against hepatitis B for up to at least 20 y after vaccination.
Keywords: challenge dose; hepatitis B; hepatitis B virus; persistence; vaccine.
Figures
Similar articles
-
Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers.Hum Vaccin Immunother. 2012 Jul;8(7):896-904. doi: 10.4161/hv.19989. Epub 2012 Jul 1. Hum Vaccin Immunother. 2012. PMID: 22777097 Free PMC article. Clinical Trial.
-
Significance and anamnestic response in isolated hepatitis B core antibody-positive individuals 18 years after neonatal hepatitis B virus vaccination in Taiwan.Vaccine. 2012 Jun 8;30(27):4034-9. doi: 10.1016/j.vaccine.2012.04.031. Epub 2012 Apr 22. Vaccine. 2012. PMID: 22531558
-
The persistence of anti-HBs antibody and anamnestic response 20 years after primary vaccination with recombinant hepatitis B vaccine at infancy.Hum Vaccin Immunother. 2014;10(12):3731-6. doi: 10.4161/hv.34393. Hum Vaccin Immunother. 2014. PMID: 25483689 Free PMC article.
-
Persistence of antibodies 20 y after vaccination with a combined hepatitis A and B vaccine.Hum Vaccin Immunother. 2017 May 4;13(5):972-980. doi: 10.1080/21645515.2016.1274473. Epub 2017 Mar 10. Hum Vaccin Immunother. 2017. PMID: 28281907 Free PMC article.
-
Hepatitis B Vaccination: A Historical Overview with a Focus on the Italian Achievements.Viruses. 2022 Jul 11;14(7):1515. doi: 10.3390/v14071515. Viruses. 2022. PMID: 35891495 Free PMC article. Review.
Cited by
-
Protection Provided by Hepatitis B Vaccine in Adult Population of Chaharmahal and Bakhtiari Province, Iran in 2013.J Clin Diagn Res. 2016 Apr;10(4):LC01-4. doi: 10.7860/JCDR/2016/17758.7548. Epub 2016 Apr 1. J Clin Diagn Res. 2016. PMID: 27190834 Free PMC article.
-
Serological evidence of hepatitis A, B, and C virus infection in older adults in Khon Kaen, Thailand and the estimated rates of chronic hepatitis B and C virus infection in Thais, 2017.PeerJ. 2019 Aug 19;7:e7492. doi: 10.7717/peerj.7492. eCollection 2019. PeerJ. 2019. PMID: 31489265 Free PMC article.
-
Long-term Immunity Against Hepatitis B Virus After Routine Immunization Among Adults Visiting Primary Care Centers in Riyadh, Saudi Arabia.Cureus. 2022 Jan 15;14(1):e21266. doi: 10.7759/cureus.21266. eCollection 2022 Jan. Cureus. 2022. PMID: 35178320 Free PMC article.
-
The Success of a Universal Hepatitis B Immunization Program as Part of Thailand's EPI after 22 Years' Implementation.PLoS One. 2016 Mar 3;11(3):e0150499. doi: 10.1371/journal.pone.0150499. eCollection 2016. PLoS One. 2016. PMID: 26938736 Free PMC article.
-
Prevention of Perinatal Hepatitis B Virus Transmission.J Pediatric Infect Dis Soc. 2014 Sep;3 Suppl 1(Suppl 1):S7-S12. doi: 10.1093/jpids/piu064. J Pediatric Infect Dis Soc. 2014. PMID: 25232477 Free PMC article.
References
-
- World Health Organization. Hepatitis B Vaccines. Wkly Epidemiol Rec 2009; 84:405-20. Available at: http://www.who.int/wer. Last accessed 26 February 2013.
-
- Heron L, Selnikova O, Moiseieva A, Van Damme P, van der Wielen M, Levie K, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11-15 years: a randomised controlled trial. Vaccine. 2007;25:2817–22. doi: 10.1016/j.vaccine.2006.12.021. - DOI - PubMed
-
- Centers for Disease Control and Prevention (CDC) Global progress toward universal childhood hepatitis B vaccination, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:868–70. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
