Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand
- PMID: 23732904
- PMCID: PMC3906265
- DOI: 10.4161/hv.24844
Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand
Abstract
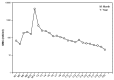
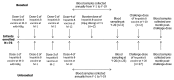
Hepatitis B vaccine has been available worldwide since the mid-1980s. This vaccine was evaluated in a clinical trial in Thailand, conducted on subjects born to hepatitis B surface antigen positive and hepatitis B e-antigen positive mothers and vaccinated according to a 4-dose schedule at 0, 1, 2 and 12 mo of age and a single dose of hepatitis B immunoglobulin concomitantly at birth. All enrolled subjects seroconverted and were followed for 20 y to assess the persistence of antibody to the hepatitis B surface antigen (anti-HBs) (NCT00240539). At year 20, 64% of subjects had anti-HBs antibody concentrations≥10 milli-international units per milli liter (mIU/ml) and 92% of subjects had detectable levels (≥3.3 mIU/ml) of anti-HBs antibodies. At year 20, subjects with anti-HBs antibody titer<100 mIU/ml were offered an additional dose of hepatitis B virus (HBV) vaccine to assess immune memory (NCT00657657). Anamnestic response to the challenge dose was observed in 96.6% of subjects with an 82-fold (13.2 to 1082.4 mIU/ml) increase in anti-HBs antibody geometric mean concentrations. This study confirms the long-term immunogenicity of the 4-dose regimen of the HBV vaccine eliciting long-term persistence of antibodies and immune memory against hepatitis B for up to at least 20 y after vaccination.
Keywords: challenge dose; hepatitis B; hepatitis B virus; persistence; vaccine.
Figures
References
-
- World Health Organization. Hepatitis B Vaccines. Wkly Epidemiol Rec 2009; 84:405-20. Available at: http://www.who.int/wer. Last accessed 26 February 2013.
-
- Heron L, Selnikova O, Moiseieva A, Van Damme P, van der Wielen M, Levie K, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11-15 years: a randomised controlled trial. Vaccine. 2007;25:2817–22. doi: 10.1016/j.vaccine.2006.12.021. - DOI - PubMed
-
- Centers for Disease Control and Prevention (CDC) Global progress toward universal childhood hepatitis B vaccination, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:868–70. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
