Single quantum dot tracking reveals that an individual multivalent HIV-1 Tat protein transduction domain can activate machinery for lateral transport and endocytosis
- PMID: 23732912
- PMCID: PMC3719669
- DOI: 10.1128/MCB.01717-12
Single quantum dot tracking reveals that an individual multivalent HIV-1 Tat protein transduction domain can activate machinery for lateral transport and endocytosis
Abstract
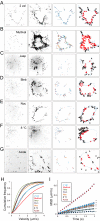
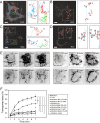
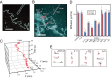
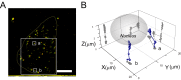
The mechanisms underlying the cellular entry of the HIV-1 Tat protein transduction domain (TatP) and the molecular information necessary to improve the transduction efficiency of TatP remain unclear due to the technical limitations for direct visualization of TatP's behavior in cells. Using confocal microscopy, total internal reflection fluorescence microscopy, and four-dimensional microscopy, we developed a single-molecule tracking assay for TatP labeled with quantum dots (QDs) to examine the kinetics of TatP initially and immediately before, at the beginning of, and immediately after entry into living cells. We report that even when the number of multivalent TatP (mTatP)-QDs bound to a cell was low, each single mTatP-QD first locally induced the cell's lateral transport machinery to move the mTatP-QD toward the center of the cell body upon cross-linking of heparan sulfate proteoglycans. The centripetal and lateral movements were linked to the integrity and flow of actomyosin and microtubules. Individual mTatP underwent lipid raft-mediated temporal confinement, followed by complete immobilization, which ultimately led to endocytotic internalization. However, bivalent TatP did not sufficiently promote either cell surface movement or internalization. Together, these findings provide clues regarding the mechanisms of TatP cell entry and indicate that increasing the valence of TatP on nanoparticles allows them to behave as cargo delivery nanomachines.
Figures


Similar articles
-
Depicting Binding-Mediated Translocation of HIV-1 Tat Peptides in Living Cells with Nanoscale Pens of Tat-Conjugated Quantum Dots.Sensors (Basel). 2017 Feb 10;17(2):315. doi: 10.3390/s17020315. Sensors (Basel). 2017. PMID: 28208588 Free PMC article.
-
Single particle tracking confirms that multivalent Tat protein transduction domain-induced heparan sulfate proteoglycan cross-linkage activates Rac1 for internalization.J Biol Chem. 2011 Mar 25;286(12):10581-92. doi: 10.1074/jbc.M110.187450. Epub 2011 Jan 3. J Biol Chem. 2011. PMID: 21199870 Free PMC article.
-
Exploring transduction mechanisms of protein transduction domains (PTDs) in living cells utilizing single-quantum dot tracking (SQT) technology.Sensors (Basel). 2012;12(1):549-72. doi: 10.3390/s120100549. Epub 2012 Jan 5. Sensors (Basel). 2012. PMID: 22368485 Free PMC article. Review.
-
Branched dimerization of Tat peptide improves permeability to HeLa and hippocampal neuronal cells.Chem Commun (Camb). 2015 Mar 28;51(25):5463-6. doi: 10.1039/c5cc00882d. Chem Commun (Camb). 2015. PMID: 25733181
-
The host cytoskeleton: a key regulator of early HIV-1 infection.FEBS J. 2024 May;291(9):1835-1848. doi: 10.1111/febs.16706. Epub 2022 Dec 26. FEBS J. 2024. PMID: 36527282 Free PMC article. Review.
Cited by
-
Live-cell single-molecule labeling and analysis of myosin motors with quantum dots.Mol Biol Cell. 2017 Jan 1;28(1):173-181. doi: 10.1091/mbc.E16-06-0413. Epub 2016 Nov 9. Mol Biol Cell. 2017. PMID: 28035048 Free PMC article.
-
Ligand Separation on Nanoconstructs Affects Targeting Selectivity to Protein Dimers on Cell Membranes.Nano Lett. 2024 Jan 10;24(1):519-524. doi: 10.1021/acs.nanolett.3c04641. Epub 2023 Dec 21. Nano Lett. 2024. PMID: 38126338 Free PMC article.
-
Depicting Binding-Mediated Translocation of HIV-1 Tat Peptides in Living Cells with Nanoscale Pens of Tat-Conjugated Quantum Dots.Sensors (Basel). 2017 Feb 10;17(2):315. doi: 10.3390/s17020315. Sensors (Basel). 2017. PMID: 28208588 Free PMC article.
-
Photothermal raster image correlation spectroscopy of gold nanoparticles in solution and on live cells.R Soc Open Sci. 2015 Jun 17;2(6):140454. doi: 10.1098/rsos.140454. eCollection 2015 Jun. R Soc Open Sci. 2015. PMID: 26543570 Free PMC article.
-
Imaging therapeutic peptide transport across intestinal barriers.RSC Chem Biol. 2021 Jun 15;2(4):1115-1143. doi: 10.1039/d1cb00024a. eCollection 2021 Aug 5. RSC Chem Biol. 2021. PMID: 34458827 Free PMC article. Review.
References
-
- Frankel AD, Pabo CO. 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189–1193 - PubMed
-
- Green M, Loewenstein PM. 1988. Autonomous functional domains of chemically synthesized human immunodeficiency virus Tat trans-activator protein. Cell 55:1179–1188 - PubMed
-
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. 1998. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4:1449–1452 - PubMed
-
- Vives E, Brodin P, Lebleu B. 1997. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272:16010–16017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
