Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease
- PMID: 23732990
- PMCID: PMC3776643
- DOI: 10.1038/mt.2013.96
Intrapleural administration of AAV9 improves neural and cardiorespiratory function in Pompe disease
Abstract
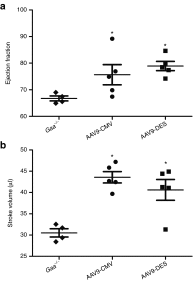
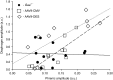
Pompe disease is a neuromuscular disease resulting from deficiency in acid α-glucosidase (GAA), results in cardiac, skeletal muscle, and central nervous system (CNS) pathology. Enzyme replacement therapy (ERT) has been shown to partially correct cardiac and skeletal muscle dysfunction. However, ERT does not cross the blood-brain barrier and progressive CNS pathology ensues. We tested the hypothesis that intrapleural administration of recombinant adeno-associated virus (rAAV9)-GAA driven by a cytomegalovirus (CMV) or desmin (DES) promoter would improve cardiac and respiratory function in Gaa(-/-) mice through a direct effect and retrograde transport to motoneurons. Cardiac magnetic resonance imaging revealed significant improvement in ejection fraction in rAAV9-GAA-treated animals. Inspiratory phrenic and diaphragm activity was examined at baseline and during hypercapnic respiratory challenge. Mice treated with AAV9 had greater relative inspiratory burst amplitude during baseline conditions when compared with Gaa(-/-). In addition, efferent phrenic burst amplitude was significantly correlated with diaphragm activity in both AAV9-DES and AAV9-CMV groups but not in Gaa(-/-). This is the first study to indicate improvements in cardiac, skeletal muscle, and respiratory neural output following rAAV administration in Pompe disease. These results further implicate a role for the CNS in Pompe disease pathology and the critical need to target the neurologic aspects in developing therapeutic strategies.
Figures


References
-
- Byrne BJ, Kishnani PS, Case LE, Merlini L, Müller-Felber W, Prasad S, et al. Pompe disease: design, methodology, and early findings from the Pompe Registry. Mol Genet Metab. 2011;103:1–11. - PubMed
-
- Burrow TA, Hopkin RJ, Leslie ND, Tinkle BT, Grabowski GA. Enzyme reconstitution/replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2007;19:628–635. - PubMed
-
- Fukuda T, Roberts A, Plotz PH, Raben N. Acid alpha-glucosidase deficiency (Pompe disease). Curr Neurol Neurosci Rep. 2007;7:71–77. - PubMed
-
- Geel TM, McLaughlin PM, de Leij LF, Ruiters MH, Niezen-Koning KE. Pompe disease: current state of treatment modalities and animal models. Mol Genet Metab. 2007;92:299–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

