ADAMTS13 predicts renal and cardiovascular events in type 2 diabetic patients and response to therapy
- PMID: 23733198
- PMCID: PMC3781447
- DOI: 10.2337/db13-0530
ADAMTS13 predicts renal and cardiovascular events in type 2 diabetic patients and response to therapy
Abstract
In patients with diabetes, impaired ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) proteolysis of highly thrombogenic von Willebrand factor (VWF) multimers may accelerate renal and cardiovascular complications. Restoring physiological VWF handling might contribute to ACE inhibitors' (ACEi) reno- and cardioprotective effects. To assess how Pro618Ala ADAMTS13 variants and related proteolytic activity interact with ACEi therapy in predicting renal and cardiovascular complications, we genotyped 1,163 normoalbuminuric type 2 diabetic patients from BErgamo NEphrologic DIabetes Complications Trial (BENEDICT). Interaction between Pro618Ala and ACEi was significant in predicting both renal and combined renal and cardiovascular events. The risk for renal or combined events versus reference Ala carriers on ACEi progressively increased from Pro/Pro homozygotes on ACEi (hazard ratio 2.80 [95% CI 0.849-9.216] and 1.58 [0.737-3.379], respectively) to Pro/Pro homozygotes on non-ACEi (4.77 [1.484-15.357] and 1.99 [0.944-4.187]) to Ala carriers on non-ACEi (8.50 [2.416-29.962] and 4.00 [1.739-9.207]). In a substudy, serum ADAMTS13 activity was significantly lower in Ala carriers than in Pro/Pro homozygotes and in case subjects with renal, cardiovascular, or combined events than in diabetic control subjects without events. ADAMTS13 activity significantly and negatively correlated with all outcomes. In patients with diabetes, ADAMTS13 618Ala variant associated with less proteolytic activity, higher risk of chronic complications, and better response to ACEi therapy. Screening for Pro618Ala polymorphism may help identify patients with diabetes at highest risk who may benefit the most from early reno- and cardioprotective therapy.
Trial registration: ClinicalTrials.gov NCT00235014.
Figures
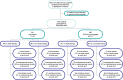
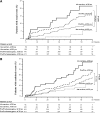


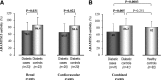
Comment in
-
Can ADAMTS13 lead us to the paradise of personalized medicine?Diabetes. 2013 Oct;62(10):3331-2. doi: 10.2337/db13-1055. Diabetes. 2013. PMID: 24065791 Free PMC article. No abstract available.
References
-
- Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008;117:1945–1954 - PubMed
-
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–232 - PubMed
-
- Ruggenenti P, Fassi A, Ilieva AP, et al. BENEDICT-B Study Investigators Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. J Hypertens 2011;29:207–216 - PubMed
-
- Ruggenenti P, Fassi A, Ilieva AP, et al. Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004;351:1941–1951 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, et al. RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–869 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

