Duloxetine use in chronic painful conditions--individual patient data responder analysis
- PMID: 23733529
- PMCID: PMC4302330
- DOI: 10.1002/j.1532-2149.2013.00341.x
Duloxetine use in chronic painful conditions--individual patient data responder analysis
Abstract
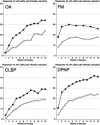
Background: Duloxetine has been studied in four distinct chronic pain conditions - osteoarthritis (OA), fibromyalgia, chronic low back pain (CLBP) and diabetic peripheral neuropathic pain (DPNP). These trials have involved large numbers of patients with at least moderate pain, and have used similar methods for recording pain intensity, over about 12 weeks.
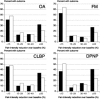
Methods: Data from the trials were pooled according to painful condition, and reanalysed at the level of the individual patient and using increasing levels of pain intensity reduction (<15%, 15-29%, 30-49%, ≥ 50%), with different imputation methods on withdrawal.
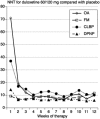
Results: The proportion of patients recording at least 50% pain intensity reduction plateaued after 2-6 weeks in fibromyalgia, and 8-12 weeks in other conditions. The duloxetine-specific benefit [number needed to treat (NNT) for at least 50% pain intensity reduction] was fairly constant after about 2 weeks for DPNP and fibromyalgia and after about 4 or 5 weeks for OA and CLBP. In all conditions, responses were bimodal, with patients generally experiencing either very good or very poor pain relief. Last-observation-carried-forward imputation produced numerically and occasionally statistically better (lower) NNTs than use of baseline-observation-carried-forward (true response).
Conclusions: Baseline-observation-carried-forward (true response), which combines the success of high levels of pain relief with the failure to experience pain relief on withdrawal of the drug is conservative and probably reflective of clinical practice experience. The distribution of effect was not normal; few patients had the average response and averages are not an appropriate descriptor for these data.
© 2013 The Authors. European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation - EFIC®.
Figures
References
-
- Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, Goldstein DJ. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–2984. - PubMed
-
- Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, Wernicke JF. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119:5–15. - PubMed
-
- Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs. pregabalin in painful diabetic neuropathy: A randomized double blind clinical trial. Diabet Med. 2009;26:1019–1026. - PubMed
-
- Bradley LA, Wohlreich MM, Wang F, Gaynor PJ, Robinson MJ, D'Souza DN, Mease PJ. Pain response profile of patients with fibromyalgia treated with duloxetine. Clin J Pain. 2010;26:498–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

