A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor
- PMID: 23733618
- PMCID: PMC4308727
- DOI: 10.1002/cncr.28142
A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor
Abstract
Background: Both everolimus and temozolomide are associated with single-agent activity in patients with pancreatic neuroendocrine tumor (NET). A phase 1/2 study was performed to evaluate the safety and efficacy of temozolomide in combination with everolimus in patients who have advanced pancreatic NET.
Methods: Patients were treated with temozolomide at a dose of 150 mg/m(2) per day on days 1 through 7 and days 15 through 21 in combination with everolimus daily in each 28-day cycle. In cohort 1, temozolomide was administered together with everolimus at 5 mg daily. Following demonstration of safety in this cohort, subsequent patients in cohort 2 were treated with temozolomide plus everolimus at 10 mg daily. The duration of temozolomide treatment was limited to 6 months. Patients were followed for toxicity, radiologic and biochemical response, and survival.
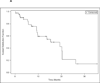
Results: A total of 43 patients were enrolled, including 7 in cohort 1 and 36 in cohort 2. Treatment was associated with known toxicities of each drug; no synergistic toxicities were observed. Among 40 evaluable patients, 16 (40%) experienced a partial response. The median progression-free survival duration was 15.4 months. Median overall survival was not reached.
Conclusions: Temozolomide and everolimus can be safely administered together in patients with advanced pancreatic NET, and the combination is associated with encouraging antitumor activity. Future studies evaluating the efficacy of combination therapy compared to treatment with either agent alone are warranted.
Keywords: everolimus; oncology; pancreatic neuroendocrine tumor; phase 1/2 study; temozolomide.
Copyright © 2013 American Cancer Society.
Figures
References
-
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. - PubMed
-
- Moertel C, Lefkopoulo M, Lipsitz S, Hahn R, Klaassen D. Streptozocin-doxorubicin, stretpozocin-fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources