Lung parenchymal mechanics
- PMID: 23733644
- PMCID: PMC3929318
- DOI: 10.1002/cphy.c100033
Lung parenchymal mechanics
Abstract
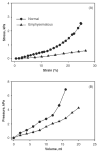
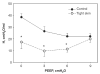
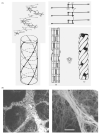
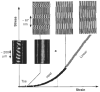
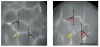
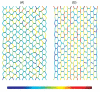
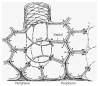
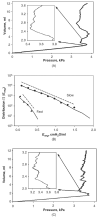
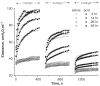
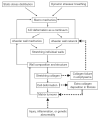
The lung parenchyma comprises a large number of thin-walled alveoli, forming an enormous surface area, which serves to maintain proper gas exchange. The alveoli are held open by the transpulmonary pressure, or prestress, which is balanced by tissues forces and alveolar surface film forces. Gas exchange efficiency is thus inextricably linked to three fundamental features of the lung: parenchymal architecture, prestress, and the mechanical properties of the parenchyma. The prestress is a key determinant of lung deformability that influences many phenomena including local ventilation, regional blood flow, tissue stiffness, smooth muscle contractility, and alveolar stability. The main pathway for stress transmission is through the extracellular matrix. Thus, the mechanical properties of the matrix play a key role both in lung function and biology. These mechanical properties in turn are determined by the constituents of the tissue, including elastin, collagen, and proteoglycans. In addition, the macroscopic mechanical properties are also influenced by the surface tension and, to some extent, the contractile state of the adherent cells. This chapter focuses on the biomechanical properties of the main constituents of the parenchyma in the presence of prestress and how these properties define normal function or change in disease. An integrated view of lung mechanics is presented and the utility of parenchymal mechanics at the bedside as well as its possible future role in lung physiology and medicine are discussed.
© 2011 American Physiological Society.
Figures








References
-
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. - PubMed
-
- Agostoni E, D'Angelo E, Bonanni MV. The effect of the abdomen on the vertical gradient of pleural surface pressure. Respir Physiol. 1970;8:332–346. - PubMed
-
- Al Jamal R, Roughley PJ, Ludwig MS. Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am J Physiol Lung Cell Mol Physiol. 2001;280:L306–L315. - PubMed
-
- Alencar AM, Arold SP, Buldyrev SV, Majumdar A, Stamenovic D, Stanley HE, Suki B. Physiology: Dynamic instabilities in the inflating lung. Nature. 2002;417:809–811. - PubMed
-
- Allen GB, Leclair T, Cloutier M, Thompson-Figueroa J, Bates JH. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1580–1589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

