Genomics and genetics in the biology of adaptation to exercise
- PMID: 23733655
- PMCID: PMC3938186
- DOI: 10.1002/cphy.c100059
Genomics and genetics in the biology of adaptation to exercise
Abstract
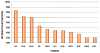
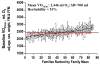
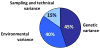
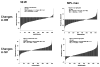
This article is devoted to the role of genetic variation and gene-exercise interactions in the biology of adaptation to exercise. There is evidence from genetic epidemiology research that DNA sequence differences contribute to human variation in physical activity level, cardiorespiratory fitness in the untrained state, cardiovascular and metabolic response to acute exercise, and responsiveness to regular exercise. Methodological and technological advances have made it possible to undertake the molecular dissection of the genetic component of complex, multifactorial traits, such as those of interest to exercise biology, in terms of tissue expression profile, genes, and allelic variants. The evidence from animal models and human studies is considered. Data on candidate genes, genome-wide linkage results, genome-wide association findings, expression arrays, and combinations of these approaches are reviewed. Combining transcriptomic and genomic technologies has been shown to be more powerful as evidenced by the development of a recent molecular predictor of the ability to increase VO2max with exercise training. For exercise as a behavior and physiological fitness as a state to be major players in public health policies will require that the role of human individuality and the influence of DNA sequence differences be understood. Likewise, progress in the use of exercise in therapeutic medicine will depend to a large extent on our ability to identify the favorable responders for given physiological properties to a given exercise regimen.
© 2011 American Physiological Society.
Figures





References
-
- Ahmetov, Druzhevskaya AM, Astratenkova IV, Popov DV, Vinogradova OL, Rogozkin VA. The ACTN3 R577X polymorphism in Russian endurance athletes. Br J Sports Med. 2010;44:649–652. - PubMed
-
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;19:716–723.
-
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

