Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer
- PMID: 23733761
- PMCID: PMC5705191
- DOI: 10.1200/JCO.2012.46.2408
Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer
Erratum in
-
Errata.J Clin Oncol. 2018 Jan 1;36(1):98. doi: 10.1200/JCO.2017.76.9976. J Clin Oncol. 2018. PMID: 29281804 Free PMC article. No abstract available.
Abstract
Purpose: Epidermal growth factor receptor is overexpressed in metastatic triple-negative breast cancers (mTNBCs), an aggressive subtype of breast cancer. Our randomized phase II study investigated cisplatin with or without cetuximab in this setting.
Patients and methods: Patients who had received no more than one previous chemotherapy regimen were randomly assigned on a 2:1 schedule to receive no more than six cycles of cisplatin plus cetuximab or cisplatin alone. Patients receiving cisplatin alone could switch to cisplatin plus cetuximab or cetuximab alone on disease progression. The primary end point was overall response rate (ORR). Secondary end points studied included progression-free survival (PFS), overall survival (OS), and safety profiles. Analyses included a significance level of α = .10 with no adjustments for multiplicity.
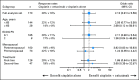
Results: The full analysis set comprised 115 patients receiving cisplatin plus cetuximab and 58 receiving cisplatin alone; 31 patients whose disease progressed on cisplatin alone switched to cetuximab-containing therapy. The ORR was 20% (95% CI, 13 to 29) with cisplatin plus cetuximab and 10% (95% CI, 4 to 21) with cisplatin alone (odds ratio, 2.13; 95% CI, 0.81 to 5.59; P = .11). Cisplatin plus cetuximab resulted in longer PFS compared with cisplatin alone (median, 3.7 v 1.5 months; hazard ratio [HR], 0.67; 95% CI, 0.47 to 0.97; P = .032). Corresponding median OS was 12.9 versus 9.4 months (HR, 0.82; 95% CI, 0.56 to 1.20; P = .31). Common grade 3/4 adverse events included acne-like rash, neutropenia, and fatigue.
Conclusion: While the primary study end point was not met, adding cetuximab to cisplatin doubled the ORR and appeared to prolong PFS and OS, warranting further investigation in mTNBC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Cheang MC Voduc D Bajdik C , etal: Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype Clin Cancer Res 14: 1368– 1376,2008. - PubMed
-
- Dent R Trudeau M Pritchard KI , etal: Triple-negative breast cancer: Clinical features and patterns of recurrence Clin Cancer Res 13: 4429– 4434,2007. - PubMed
-
- Kassam F Enright K Dent R , etal: Survival outcomes for patients with metastatic triple-negative breast cancer: Implications for clinical practice and trial design Clin Breast Cancer 9: 29– 33,2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

