Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database
- PMID: 23733765
- PMCID: PMC3699725
- DOI: 10.1200/JCO.2013.49.6638
Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database
Abstract
Purpose: Prior studies have suggested that patients with stage II/III colon cancer receive similar benefit from intravenous (IV) fluoropyrimidine adjuvant therapy regardless of age. Combination regimens and oral fluorouracil (FU) therapy are now standard. We examined the impact of age on colon cancer recurrence and mortality after adjuvant therapy with these newer options.
Patients and methods: We analyzed 11,953 patients age < 70 and 2,575 age ≥ 70 years from seven adjuvant therapy trials comparing IV FU with oral fluoropyrimidines (capecitabine, uracil, or tegafur) or combinations of fluoropyrimidines with oxaliplatin or irinotecan in stage II/III colon cancer. End points were disease-free survival (DFS), overall survival (OS), and time to recurrence (TTR).
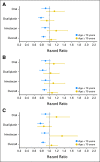
Results: In three studies comparing oxaliplatin-based chemotherapy with IV FU, statistically significant interactions were not observed between treatment arm and age (P interaction = .09 for DFS, .05 for OS, and .36 for TTR), although the stratified point estimates suggested limited benefit from the addition of oxaliplatin in elderly patients (DFS hazard ratio [HR], 0.94; 95% CI, 0.78 to 1.13; OS HR, 1.04; 95% CI, 0.85 to 1.27). No significant interactions by age were detected with oral fluoropyrimidine therapy compared with IV FU; noninferiority was supported in both age populations.
Conclusion: Patients age ≥ 70 years seemed to experience reduced benefit from adding oxaliplatin to fluoropyrimidines in the adjuvant setting, although statistically, there was not a significant effect modification by age, whereas oral fluoropyrimidines retained their efficacy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. - PubMed
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Pasetto LM, Monfardini S. Colorectal cancer screening in elderly patients: When should be more useful? Cancer Treat Rev. 2007;33:528–532. - PubMed
-
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. - PubMed
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

