Small molecule inhibitors of trans-translation have broad-spectrum antibiotic activity
- PMID: 23733947
- PMCID: PMC3690859
- DOI: 10.1073/pnas.1302816110
Small molecule inhibitors of trans-translation have broad-spectrum antibiotic activity
Abstract
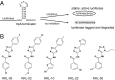
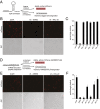
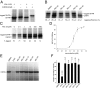
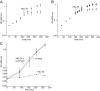
The trans-translation pathway for protein tagging and ribosome release plays a critical role for viability and virulence in a wide range of pathogens but is not found in animals. To explore the use of trans-translation as a target for antibiotic development, a high-throughput screen and secondary screening assays were used to identify small molecule inhibitors of the pathway. Compounds that inhibited protein tagging and proteolysis of tagged proteins were recovered from the screen. One of the most active compounds, KKL-35, inhibited the trans-translation tagging reaction with an IC50 = 0.9 µM. KKL-35 and other compounds identified in the screen exhibited broad-spectrum antibiotic activity, validating trans-translation as a target for drug development. This unique target could play a key role in combating strains of pathogenic bacteria that are resistant to existing antibiotics.
Keywords: antibiotic target; non-stop translation; tmRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Infectious Diseases Society of America The 10 x ’20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50(8):1081–1083. - PubMed
-
- Keiler KC. Biology of trans-translation. Annu Rev Microbiol. 2008;62:133–151. - PubMed
-
- Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem. 2007;76:101–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

