A novel GUCY2D mutation in a Chinese family with dominant cone dystrophy
- PMID: 23734073
- PMCID: PMC3668702
A novel GUCY2D mutation in a Chinese family with dominant cone dystrophy
Abstract
Purpose: To describe the clinical and genetic findings in a Chinese family with autosomal dominant cone dystrophy (adCOD).
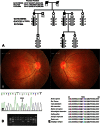
Methods: One family was examined clinically, and genomic DNA was extracted from venous blood of all participants. Genotyping and haplotyping analysis was performed on the known genetic loci for adCOD and autosomal dominant cone-rod dystrophies (adCORD) with a panel of polymorphic markers in this family. All coding exons of the AIPL1, PTTPNM3, and GUCY2D gene were directly sequenced. Allele-specific PCR was used to validate a substitution in all available family members and 100 normal controls. Bioinformatics analysis was done using the Garnier-Osguthorpe-Robson method to predict the effect of the variants detected on the secondary structure of the GUCY2D protein.
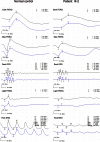
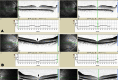
Results: Clinical examination and pedigree analysis revealed a three-generation family with four members diagnosed with adCOD. Through genotyping, the disease-causing genes were mapped to chromosomes 17p13.1-2 (AIPL1, PITPNM3, and GUCY2D gene). A novel A->G transition at position 2545 (p.T849A) of the cDNA sequence was identified in the GUCY2D gene. No mutation was detected in the AIPL1 and PITPNM3 genes. This missense mutation co-segregated with the disease phenotype of the family but was not found in the 100 normal controls.
Conclusions: A novel missense mutation of the GUCY2D gene was identified in this study. Our results further confirm that the dimerization zone of RetGC-1 is the mutational hot region for COD and CORD.
Figures
References
-
- Kelsell RE, Gregory-Evans K, Payne A, Perrault I, Kaplan J, Yang RB, Garbers DL, Bird AC, Moore AT, Hunt DM. Mutations in the retinal guanylate cyclase (RETGC-1) gene in dominant cone-rod dystrophy. Hum Mol Genet. 1998;7:1179–84. - PubMed
-
- Gregory-Evans K, Kelsell R, Gregory-Evans C, Downes SM, Fitzke FM, Holder GR, Simunovic M, Mollon JD, Taylor R, Hunt DM, Bird AC, Moore AT. Autosomal dominant cone rod retinal dystrophy (CORD6) from heterozygous mutation of GUCY2D, which encodes retinal guanylate cyclase. Ophthalmology. 2000;107:55–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
