Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy
- PMID: 23734074
- PMCID: PMC3668662
Retinal O-linked N-acetylglucosamine protein modifications: implications for postnatal retinal vascularization and the pathogenesis of diabetic retinopathy
Abstract
Purpose: Hyperglycemia activates several metabolic pathways, including the hexosamine biosynthetic pathway. Uridine diphosphate N-acetylglucosamine (GlcNAc) is the product of the hexosamine biosynthetic pathway and the substrate for O-linked GlcNAc (O-GlcNAc) modification. This modification affects a wide range of proteins by altering their activity, cellular localization, and/or protein interactions. However, the role O-GlcNAcylation may play in normal postnatal retinal vascular development and in the ocular complications of diabetes, including diabetic retinopathy, requires further investigation.
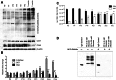
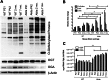
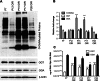
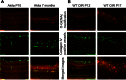
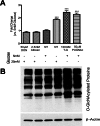
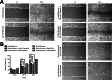
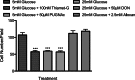
Methods: The total levels of O-GlcNAc-modified proteins were evaluated by western blot analysis of lysates prepared from retinas obtained at different days during postnatal retinal vascularization and oxygen-induced ischemic retinopathy. Similar experiments were performed with retinal lysate prepared from diabetic Ins2(Akita/+) mice with different durations of diabetes and retinal vascular cells cultured under various glucose conditions. The localization of O-GlcNAc-modified proteins in the retinal vasculature was confirmed by immunofluorescence staining. The impact of altered O-GlcNAcylation on the migration of retinal vascular cells was determined using scratch wound and transwell migration assays.
Results: We detected an increase in protein O-GlcNAcylation during mouse postnatal retinal vascularization and aging, in part through the regulation of the enzymes that control this modification. The study of the diabetic Ins2(Akita/+) mouse retina showed an increase in the O-GlcNAc modification of retinal proteins. We also observed an increase in retinal O-GlcNAcylated protein levels during the neovascularization phase of oxygen-induced ischemic retinopathy. Our fluorescence microscopy data confirmed that the alterations in retinal O-GlcNAcylation are similarly represented in the retinal vasculature and in retinal pericytes and endothelial cells. Particularly, the migration of retinal pericytes, but not retinal endothelial cells, was attenuated by increased O-GlcNAc modification.
Conclusions: The O-GlcNAc modification pattern changes during postnatal retinal vascular development and neovascularization, and its dysregulation under hyperglycemia and/or ischemia may contribute to the pathogenesis of the diabetic retinopathy and retinal neovascularization.
Figures

References
-
- Ockrim Z, Yorston D. Managing diabetic retinopathy. BMJ. 2010;341:c5400. - PubMed
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36. - PubMed
-
- Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353:839–41. - PubMed
-
- Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
