Transgenic mouse lines for non-invasive ratiometric monitoring of intracellular chloride
- PMID: 23734096
- PMCID: PMC3659292
- DOI: 10.3389/fnmol.2013.00011
Transgenic mouse lines for non-invasive ratiometric monitoring of intracellular chloride
Abstract
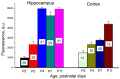
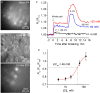
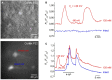
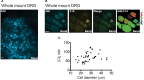
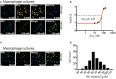
Chloride is the most abundant physiological anion and participates in a variety of cellular processes including trans-epithelial transport, cell volume regulation, and regulation of electrical excitability. The development of tools to monitor intracellular chloride concentration ([Cli]) is therefore important for the evaluation of cellular function in normal and pathological conditions. Recently, several Cl-sensitive genetically encoded probes have been described which allow for non-invasive monitoring of [Cli]. Here we describe two mouse lines expressing a CFP-YFP-based Cl probe called Cl-Sensor. First, we generated transgenic mice expressing Cl-Sensor under the control of the mouse Thy1 mini promoter. Cl-Sensor exhibited good expression from postnatal day two (P2) in neurons of the hippocampus and cortex, and its level increased strongly during development. Using simultaneous whole-cell monitoring of ionic currents and Cl-dependent fluorescence, we determined that the apparent EC 50 for Cli was 46 mM, indicating that this line is appropriate for measuring neuronal [Cli] in postnatal mice. We also describe a transgenic mouse reporter line for Cre-dependent conditional expression of Cl-Sensor, which was targeted to the Rosa26 locus and by incorporating a strong exogenous promoter induced robust expression upon Cre-mediated recombination. We demonstrate high levels of tissue-specific expression in two different Cre-driver lines targeting cells of the myeloid lineage and peripheral sensory neurons. Using these mice the apparent EC 50 for Cli was estimated to be 61 and 54 mM in macrophages and DRG, respectively. Our data suggest that these mouse lines will be useful models for ratiometric monitoring of Cli in specific cell types in vivo.
Keywords: brain slices; dorsal root ganglia; fluorescent biosensors; intracellular chloride; macrophages; non-invasive monitoring; optogenetics.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

