Dysregulated proinflammatory and fibrogenic phenotype of fibroblasts in cystic fibrosis
- PMID: 23734196
- PMCID: PMC3667188
- DOI: 10.1371/journal.pone.0064341
Dysregulated proinflammatory and fibrogenic phenotype of fibroblasts in cystic fibrosis
Abstract
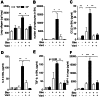
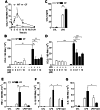
Morbi-mortality in cystic fibrosis (CF) is mainly related to chronic lung infection and inflammation, uncontrolled tissue rearrangements and fibrosis, and yet the underlying mechanisms remain largely unknown. We evaluated inflammatory and fibrosis responses to bleomycin in F508del homozygous and wild-type mice, and phenotype of fibroblasts explanted from mouse lungs and skin. The effect of vardenafil, a cGMP-specific phosphodiesterase type 5 inhibitor, was tested in vivo and in culture. Responses of proinflammatory and fibrotic markers to bleomycin were enhanced in lungs and skin of CF mice and were prevented by treatment with vardenafil. Purified lung and skin fibroblasts from CF mice proliferated and differentiated into myofibroblasts more prominently and displayed higher sensitivity to growth factors than those recovered from wild-type littermates. Under inflammatory stimulation, mRNA and protein expression of proinflammatory mediators were higher in CF than in wild-type fibroblasts, in which CFTR expression reached similar levels to those observed in other non-epithelial cells, such as macrophages. Increased proinflammatory responses in CF fibroblasts were reduced by half with submicromolar concentrations of vardenafil. Proinflammatory and fibrogenic functions of fibroblasts are upregulated in CF and are reduced by vardenafil. This study provides compelling new support for targeting cGMP signaling pathway in CF pharmacotherapy.
Conflict of interest statement
Figures



References
-
- Andersen DH (1938) Cystic fibrosis of the pancreas and its relation to celiac disease: a clinical and pathological study. Am J Dis Child 56: 344–399.
-
- Sciaky D, Brazer W (2000) Center DM, Cruikshank WW, Smith TJ (2000) Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol 164: 3806–3814. - PubMed
-
- Hogaboam CM, Steinhauser ML, Chensue SW, Kunkel SL (1998) Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Internat 54: 2152–2159. - PubMed
-
- Sweeney SE, Firestein GS (2004) Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol 36: 372–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

