Reporting quality of social and psychological intervention trials: a systematic review of reporting guidelines and trial publications
- PMID: 23734256
- PMCID: PMC3666983
- DOI: 10.1371/journal.pone.0065442
Reporting quality of social and psychological intervention trials: a systematic review of reporting guidelines and trial publications
Abstract
Background: Previous reviews show that reporting guidelines have improved the quality of trial reports in medicine, yet existing guidelines may not be fully suited for social and psychological intervention trials.
Objective/design: We conducted a two-part study that reviewed (1) reporting guidelines for and (2) the reporting quality of social and psychological intervention trials.
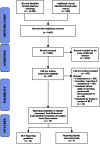
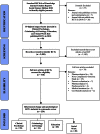
Data sources: (1) To identify reporting guidelines, we systematically searched multiple electronic databases and reporting guideline registries. (2) To identify trials, we hand-searched 40 journals with the 10 highest impact factors in clinical psychology, criminology, education, and social work. ELIGIBILITY: (1) Reporting guidelines consisted of articles introducing a checklist of reporting standards relevant to social and psychological intervention trials. (2) Trials reported randomised experiments of complex interventions with psychological, social, or health outcomes.
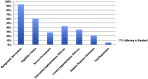
Results: (1) We identified 19 reporting guidelines that yielded 147 reporting standards relevant to social and psychological interventions. Social and behavioural science guidelines included 89 standards not found in CONSORT guidelines. However, CONSORT guidelines used more recommended techniques for development and dissemination compared to other guidelines. (2) Our review of trials (n = 239) revealed that many standards were poorly reported, such as identification as a randomised trial in titles (20% reported the information) and abstracts (55%); information about blinding (15%), sequence generation (23%), and allocation concealment (17%); and details about actual delivery of experimental (43%) and control interventions (34%), participant uptake (25%), and service environment (28%). Only 11 of 40 journals referenced reporting guidelines in "Instructions to Authors."
Conclusion: Existing reporting guidelines have important limitations in content, development, and/or dissemination. Important details are routinely missing from trial publications; most leading journals in social and behavioural sciences do not ask authors to follow reporting standards. Findings demonstrate a need to develop a CONSORT extension with updated standards for social and psychological intervention trials.
Conflict of interest statement
Figures
References
-
- Medical Research Council (2008) Developing and evaluating complex interventions: New guidance. London: MRC.
-
- Pawson R, Greenhalgh T, Harvey G, Walshe K (2004) Realist synthesis: An introduction. University of Manchester: ESRC Research Methods Programme.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical