TLR4 ligand/H₂O₂ enhances TGF-β1 signaling to induce metastatic potential of non-invasive breast cancer cells by activating non-Smad pathways
- PMID: 23734265
- PMCID: PMC3667026
- DOI: 10.1371/journal.pone.0065906
TLR4 ligand/H₂O₂ enhances TGF-β1 signaling to induce metastatic potential of non-invasive breast cancer cells by activating non-Smad pathways
Abstract
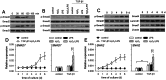
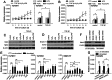
TGF-β1 has the potential to activate multiple signaling pathways required for inducing metastatic potential of tumor cells. However, TGF-β1 was inefficient in inducing metastatic potential of many non-invasive human tumor cells. Here we report that the enhancement of TGF-β1 signaling is required for inducing metastatic potential of non-invasive breast cancer cells. TGF-β1 alone could not efficiently induce the sustained activation of Smad and non-Smad pathways in non-invasive breast cancer cells. TLR4 ligand (LPS) and H₂O₂ cooperated with TGF-β1 to enhance the sustained activation of non-Smad pathways, including p38MAPK, ERK, JNK, PI3K, and NF-κB. The activation of MAPK and PI3K pathways resulted in a positive feed-back effect on TGF-β1 signaling by down-regulating Nm23-H1 expression and up-regulating the expression of TβRI and TβRII, favoring further activation of multiple signaling pathways. Moreover, the enhanced TGF-β1 signaling induced higher expression of SNAI2, which also promoted TβRII expression. Therefore, the sustained activation levels of both Smad and non-Smad pathways were gradually increased after prolonged stimulation with TGF-β1/H₂O₂/LPS. Consistent with the activation pattern of signaling pathways, the invasive capacity and anoikis-resistance of non-invasive breast cancer cells were gradually increased after prolonged stimulation with TGF-β1/H₂O₂/LPS. The metastatic potential induced by TGF-β1/H₂O₂/LPS was sufficient for tumor cells to extravasate and form metastatic foci in an experimental metastasis model in nude mice. The findings in this study suggested that the enhanced signaling is required for inducing higher metastatic capacity of tumor cells, and that targeting one of stimuli or signaling pathways might be potential approach in comprehensive strategy for tumor therapy.
Conflict of interest statement
Figures




Similar articles
-
TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways.Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L790-9. doi: 10.1152/ajplung.00099.2007. Epub 2007 Jun 29. Am J Physiol Lung Cell Mol Physiol. 2007. Retraction in: Am J Physiol Lung Cell Mol Physiol. 2012 Apr 1;302(7):L719. doi: 10.1152/ajplung.zh5-6097-retr.2012. PMID: 17601799 Free PMC article. Retracted.
-
β3 integrin promotes TGF-β1/H2O2/HOCl-mediated induction of metastatic phenotype of hepatocellular carcinoma cells by enhancing TGF-β1 signaling.PLoS One. 2013 Nov 18;8(11):e79857. doi: 10.1371/journal.pone.0079857. eCollection 2013. PLoS One. 2013. PMID: 24260309 Free PMC article.
-
Sirtuin 6 promotes transforming growth factor-β1/H2O2/HOCl-mediated enhancement of hepatocellular carcinoma cell tumorigenicity by suppressing cellular senescence.Cancer Sci. 2015 May;106(5):559-66. doi: 10.1111/cas.12632. Epub 2015 Mar 9. Cancer Sci. 2015. PMID: 25683165 Free PMC article.
-
Two faces of TGF-beta1 in breast cancer.Mediators Inflamm. 2014;2014:141747. doi: 10.1155/2014/141747. Epub 2014 May 7. Mediators Inflamm. 2014. PMID: 24891760 Free PMC article. Review.
-
Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways.Cold Spring Harb Perspect Biol. 2017 Jan 3;9(1):a022137. doi: 10.1101/cshperspect.a022137. Cold Spring Harb Perspect Biol. 2017. PMID: 27836834 Free PMC article. Review.
Cited by
-
Suppression of MD2 inhibits breast cancer in vitro and in vivo.Clin Transl Oncol. 2021 Sep;23(9):1811-1817. doi: 10.1007/s12094-021-02587-9. Epub 2021 Mar 17. Clin Transl Oncol. 2021. PMID: 33733435 Free PMC article.
-
Should a Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to treat cancer? TLR-4: its expression and effects in the ten most common cancers.Onco Targets Ther. 2013 Nov 5;6:1573-87. doi: 10.2147/OTT.S50838. Onco Targets Ther. 2013. PMID: 24235843 Free PMC article. Review.
-
Reactive Oxygen Species-Dependent Calpain Activation Contributes to Airway and Pulmonary Vascular Remodeling in Chronic Obstructive Pulmonary Disease.Antioxid Redox Signal. 2019 Oct 20;31(12):804-818. doi: 10.1089/ars.2018.7648. Epub 2019 Jun 24. Antioxid Redox Signal. 2019. PMID: 31088299 Free PMC article.
-
Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCα/ERK1/2 and PI3K/Akt pathway.Med Oncol. 2019 Nov 13;37(1):5. doi: 10.1007/s12032-019-1320-y. Med Oncol. 2019. PMID: 31720873
-
TGF-β1 and TNF-α synergistically induce epithelial to mesenchymal transition of breast cancer cells by enhancing TAK1 activation.J Cell Commun Signal. 2019 Sep;13(3):369-380. doi: 10.1007/s12079-019-00508-8. Epub 2019 Feb 9. J Cell Commun Signal. 2019. PMID: 30739244 Free PMC article.
References
-
- Gallego MI, Bierie B, Hennighausen L (2003) Targeted expression of HGF/SF in mouse mammary epithelium leads to metastatic adenosquamous carcinomas through the activation of multiple signal transduction pathways. Oncogene 22: 8498–8508. - PubMed
-
- Wu WS, Wu JR, Hu CT (2008) Signal cross talks for sustained MAPK activation and cell migration: the potential role of reactive oxygen species. Cancer Metastasis Rev 27: 303–314. - PubMed
-
- Liao SJ, Zhou YH, Yuan Y, Li D, Wu FH, et al. (2012) Triggering of Toll-like receptor 4 on metastatic breast cancer cells promotes alphavbeta3-mediated adhesion and invasive migration. Breast Cancer Res Treat 133: 853–863. - PubMed
-
- Krueger JS, Keshamouni VG, Atanaskova N, Reddy KB (2001) Temporal and quantitative regulation of mitogen-activated protein kinase (MAPK) modulates cell motility and invasion. Oncogene 20: 4209–4218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

