Electroplating and magnetostructural characterization of multisegmented Co54Ni46/Co85Ni15 nanowires from single electrochemical bath in anodic alumina templates
- PMID: 23735184
- PMCID: PMC3680049
- DOI: 10.1186/1556-276X-8-263
Electroplating and magnetostructural characterization of multisegmented Co54Ni46/Co85Ni15 nanowires from single electrochemical bath in anodic alumina templates
Abstract
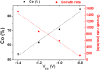
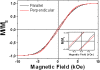
Highly hexagonally ordered hard anodic aluminum oxide membranes, which have been modified by a thin cover layer of SiO2 deposited by atomic layer deposition method, were used as templates for the synthesis of electrodeposited magnetic Co-Ni nanowire arrays having diameters of around 180 to 200 nm and made of tens of segments with alternating compositions of Co54Ni46 and Co85Ni15. Each Co-Ni single segment has a mean length of around 290 nm for the Co54Ni46 alloy, whereas the length of the Co85Ni15 segments was around 430 nm. The composition and crystalline structure of each Co-Ni nanowire segment were determined by transmission electron microscopy and selected area electron diffraction techniques. The employed single-bath electrochemical nanowire growth method allows for tuning both the composition and crystalline structure of each individual Co-Ni segment. The room temperature magnetic behavior of the multisegmented Co-Ni nanowire arrays is also studied and correlated with their structural and morphological properties.
Figures




References
-
- Arico AS, Bruce P, Scrosati B, Tarascon J-M, van Schalkwijk W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 2005;8:366–377. - PubMed
-
- Rao CNR, Deepak FL, Gundiah G, Govindaraj A. Inorganic nanowires. Progress in Solid State Chemistry. 2003;8:5–147. doi: 10.1016/j.progsolidstchem.2003.08.001. - DOI
-
- Rao CNR, Govindaraj A. Synthesis of inorganic nanotubes. Adv Mater. 2009;8:4208–4233. doi: 10.1002/adma.200803720. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

