Resistance to paclitxel in breast carcinoma cells requires a quality control of mitochondrial antiapoptotic proteins by TRAP1
- PMID: 23735188
- PMCID: PMC5528458
- DOI: 10.1016/j.molonc.2013.04.009
Resistance to paclitxel in breast carcinoma cells requires a quality control of mitochondrial antiapoptotic proteins by TRAP1
Abstract
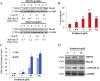
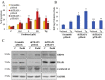
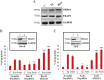
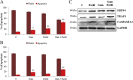
TRAP1 is a mitochondrial antiapoptotic protein up-regulated in several human malignancies. However, recent evidences suggest that TRAP1 is also localized in the endoplasmic reticulum (ER) where it is involved in ER stress protection and protein quality control of tumor cells. Based on the mechanistic link between ER stress, protection from apoptosis and drug resistance, we questioned whether these novel roles of TRAP1 are relevant for its antiapoptotic function. Here, we show for the first time that: i) TRAP1 expression is increased in about 50% of human breast carcinomas (BC), and ii) the ER stress protecting activity of TRAP1 is conserved in human tumors since TRAP1 is co-upregulated with the ER stress marker, BiP/Grp78. Notably, ER-associated TRAP1 modulates mitochondrial apoptosis by exerting a quality control on 18 kDa Sorcin, a TRAP1 mitochondrial client protein involved in TRAP1 cytoprotective pathway. Furthermore, this TRAP1 function is relevant in favoring resistance to paclitaxel, a microtubule stabilizing/ER stress inducer agent widely used in BC therapy. Indeed, the transfection of a TRAP1 deletion mutant, whose localization is restricted to the ER, in shTRAP1 cells enhances the expression of mitochondrial Sorcin and protects from apoptosis induced by ER stress agents and paclitaxel. Furthermore, BC cells adapted to paclitaxel or ER stress inducers share common resistance mechanisms: both cell models exhibit cross-resistance to single agents and the inhibition of TRAP1 by siRNAs or gamitrinib, a mitochondria-directed HSP90 family inhibitor, in paclitaxel-resistant cells rescues the sensitivity to paclitaxel. These results support the hypothesis that ER-associated TRAP1 is responsible for an extramitochondrial control of apoptosis and, therefore, an interference of ER stress adaptation through TRAP1 inhibition outside of mitochondria may be considered a further compartment-specific molecular approach to rescue drug-resistance.
Keywords: Apoptosis; Breast carcinoma; Drug resistance; ER stress; Paclitaxel; TRAP1.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures



Similar articles
-
TRAP1 role in endoplasmic reticulum stress protection favors resistance to anthracyclins in breast carcinoma cells.Int J Oncol. 2014 Feb;44(2):573-82. doi: 10.3892/ijo.2013.2199. Epub 2013 Nov 29. Int J Oncol. 2014. PMID: 24297638
-
TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins.Cell Death Differ. 2012 Apr;19(4):592-604. doi: 10.1038/cdd.2011.128. Epub 2011 Oct 7. Cell Death Differ. 2012. PMID: 21979464 Free PMC article.
-
Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents.Cancer Res. 2010 Aug 15;70(16):6577-86. doi: 10.1158/0008-5472.CAN-10-1256. Epub 2010 Jul 20. Cancer Res. 2010. PMID: 20647321
-
Heat shock proteins, cell survival and drug resistance: the mitochondrial chaperone TRAP1, a potential novel target for ovarian cancer therapy.Gynecol Oncol. 2010 May;117(2):177-82. doi: 10.1016/j.ygyno.2009.10.078. Epub 2009 Nov 25. Gynecol Oncol. 2010. PMID: 19942270 Review.
-
The Role of HSP90 and TRAP1 Targets on Treatment in Hepatocellular Carcinoma.Mol Biotechnol. 2025 Apr;67(4):1367-1381. doi: 10.1007/s12033-024-01151-4. Epub 2024 Apr 29. Mol Biotechnol. 2025. PMID: 38684604 Review.
Cited by
-
Translational control in the stress adaptive response of cancer cells: a novel role for the heat shock protein TRAP1.Cell Death Dis. 2013 Oct 10;4(10):e851. doi: 10.1038/cddis.2013.379. Cell Death Dis. 2013. PMID: 24113185 Free PMC article.
-
Nonstructural 5A Protein of Hepatitis C Virus Regulates Soluble Resistance-Related Calcium-Binding Protein Activity for Viral Propagation.J Virol. 2015 Dec 30;90(6):2794-805. doi: 10.1128/JVI.02493-15. J Virol. 2015. PMID: 26719254 Free PMC article.
-
Mitochondria: Insights into Crucial Features to Overcome Cancer Chemoresistance.Int J Mol Sci. 2021 Apr 30;22(9):4770. doi: 10.3390/ijms22094770. Int J Mol Sci. 2021. PMID: 33946271 Free PMC article. Review.
-
Ruscogenin attenuated tight junction injury and tumor migration in colorectal liver metastasis mice via regulating TRAP1.Transl Cancer Res. 2021 Mar;10(3):1470-1483. doi: 10.21037/tcr-20-2968. Transl Cancer Res. 2021. PMID: 35116472 Free PMC article.
-
Endoplasmic Reticulum Stress and Unfolded Protein Response in Breast Cancer: The Balance between Apoptosis and Autophagy and Its Role in Drug Resistance.Int J Mol Sci. 2019 Feb 16;20(4):857. doi: 10.3390/ijms20040857. Int J Mol Sci. 2019. PMID: 30781465 Free PMC article. Review.
References
-
- Amoroso, M.R. , Matassa, D.S. , Laudiero, G. , Egorova, A.V. , Polishchuk, R.S. , Maddalena, F. , Piscazzi, A. , Paladino, S. , Sarnataro, D. , Garbi, C. , Landriscina, M. , Esposito, F. , 2012. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell. Death Differ.. 19, 592–604. - PMC - PubMed
-
- Argon, Y. , Simen, B.B. , 1999. GRP94, an ER chaperone with protein and peptide binding properties. Semin. Cell. Dev. Biol.. 10, 495–505. - PubMed
-
- Badiola, N. , Penas, C. , Miñano-Molina, A. , Barneda-Zahonero, B. , Fadó, R. , Sánchez-Opazo, G. , Comella, J.X. , Sabriá, J. , Zhu, C. , Blomgren, K. , Casas, C. , Rodríguez-Alvarez, J. , 2011. Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell. Death Dis.. 2, e149 - PMC - PubMed
-
- Barone, C. , Landriscina, M. , Quirino, M. , Basso, M. , Pozzo, C. , Schinzari, G. , Di Leonardo, G. , D'Argento, E. , Trigila, N. , Cassano, A. , 2007. Schedule-dependent activity of 5-fluorouracil and irinotecan combination in the treatment of human colorectal cancer: in vitro evidence and a phase I dose-escalating clinical trial. Br. J. Cancer. 96, 21–28. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

