Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes
- PMID: 23735192
- PMCID: PMC3757534
- DOI: 10.1089/jop.2013.0009
Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes
Abstract
Purpose: To compare the pharmacokinetics (PKs) of intravitreally injected bevacizumab in vitrectomized versus nonvitrectomized control rabbit eyes.
Methods: Twenty-five-gauge pars plana vitrectomy without lensectomy was performed in 17 right rabbit eyes (V) and 18 nonvitrectomized right rabbit eyes served as controls (C). After 1.25 mg/0.05 mL intravitreal bevacizumab (IVB) injections, eyes were enucleated at 1 h, 1, 2, 5, 14, and 30 days after the injection and immediately frozen at -80°C. Bevacizumab concentrations were determined after separation of frozen vitreous and aqueous humor (AH) compartments using indirect enzyme-linked immunosorbent assay. Bevacizumab concentration-time data were analyzed to obtain PK data.
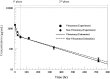
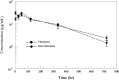
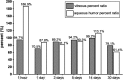
Results: Vitreous clearance of IVB consisted of 2 phases, the first fast distribution and second slow elimination phase. Clearance of IVB was accelerated in V eyes only during the first phase and not in the second phase. The vitreous concentration percent ratios between V and C eyes were 94.7% (1 h), 70.5% (1 day), 89.2% (2 days), 94.2% (5 days), 99.2% (14 days), and 79.1% (30 days). Overall vitreous half-lives were 6.99 and 7.06 days for V and C eyes, respectively (1.6-h difference).
Conclusion: Overall IVB PKs in rabbit eyes after vitrectomy without lensectomy are not substantially different from nonvitrectomized control eyes.
Figures
References
-
- Aiello L.P. Avery R.L. Arrigg P.G., et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. - PubMed
-
- Rosenfeld P.J. Brown D.M. Heier J.S., et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. - PubMed
-
- Nguyen Q.D. Brown D.M. Marcus D.M., et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. - PubMed
-
- Campochiaro P.A. Heier J.S. Feiner L., et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–1112 e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

