Transmission potential of Rift Valley fever virus over the course of the 2010 epidemic in South Africa
- PMID: 23735606
- PMCID: PMC3713830
- DOI: 10.3201/eid1906.121641
Transmission potential of Rift Valley fever virus over the course of the 2010 epidemic in South Africa
Abstract
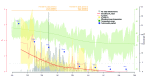
A Rift Valley fever (RVF) epidemic affecting animals on domestic livestock farms was reported in South Africa during January-August 2010. The first cases occurred after heavy rainfall, and the virus subsequently spread countrywide. To determine the possible effect of environmental conditions and vaccination on RVF virus transmissibility, we estimated the effective reproduction number (Re) for the virus over the course of the epidemic by extending the Wallinga and Teunis algorithm with spatial information. Re reached its highest value in mid-February and fell below unity around mid-March, when vaccination coverage was 7.5%-45.7% and vector-suitable environmental conditions were maintained. The epidemic fade-out likely resulted first from the immunization of animals following natural infection or vaccination. The decline in vector-suitable environmental conditions from April onwards and further vaccination helped maintain Re below unity. Increased availability of vaccine use data would enable evaluation of the effect of RVF vaccination campaigns.
Keywords: Rift Valley fever; Rift Valley fever virus; South Africa; epidemic; likelihood functions; transmission; viruses; zoonoses.
Figures

References
-
- Logan TM, Linthicum KJ, Thande PC, Wagateh JN, Nelson GO, Roberts CR. Egg hatching of Aedes mosquitoes during successive floodings in a Rift Valley fever endemic area in Kenya. J Am Mosq Control Assoc. 1991;7:109–12 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
