Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial
- PMID: 23735724
- PMCID: PMC3781531
- DOI: 10.2337/dc13-0092
Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial
Abstract
Objective: We aimed to evaluate the added value of intensive self-monitoring of blood glucose (SMBG), structured in timing and frequency, in noninsulin-treated patients with type 2 diabetes.
Research design and methods: The 12-month, randomized, clinical trial enrolled 1,024 patients with noninsulin-treated type 2 diabetes (median baseline HbA1c, 7.3% [IQR, 6.9-7.8%]) at 39 diabetes clinics in Italy. After standardized education, 501 patients were randomized to intensive structured monitoring (ISM) with 4-point glycemic profiles (fasting, preprandial, 2-h postprandial, and postabsorptive measurements) performed 3 days/week; 523 patients were randomized to active control (AC) with 4-point glycemic profiles performed at baseline and at 6 and 12 months. Two primary end points were tested in hierarchical order: HbA1c change at 12 months and percentage of patients at risk target for low and high blood glucose index.
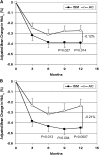
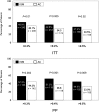
Results: Intent-to-treat analysis showed greater HbA1c reductions over 12 months in ISM (-0.39%) than in AC patients (-0.27%), with a between-group difference of -0.12% (95% CI, -0.210 to -0.024; P=0.013). In the per-protocol analysis, the between-group difference was -0.21% (-0.331 to -0.089; P=0.0007). More ISM than AC patients achieved clinically meaningful reductions in HbA1c (>0.3, >0.4, or >0.5%) at study end (P<0.025). The proportion of patients reaching/maintaining the risk target at month 12 was similar in ISM (74.6%) and AC (70.1%) patients (P=0.131). At visits 2, 3, and 4, diabetes medications were changed more often in ISM than in AC patients (P<0.001).
Conclusions: Use of structured SMBG improves glycemic control and provides guidance in prescribing diabetes medications in patients with relatively well-controlled noninsulin-treated type 2 diabetes.
Trial registration: ClinicalTrials.gov NCT00643474.
Figures

Comment in
-
Comment on: Bosi et al. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care 2013;36:2887-2894.Diabetes Care. 2013 Dec;36(12):e217. doi: 10.2337/dc13-1394. Diabetes Care. 2013. PMID: 24265386 Free PMC article. No abstract available.
-
Response to comment on: Bosi et al. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care 2013;36:2887-2894.Diabetes Care. 2013 Dec;36(12):e218. doi: 10.2337/dc13-1683. Diabetes Care. 2013. PMID: 24265387 Free PMC article. No abstract available.
References
-
- International Diabetes Federation Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Available from http://www.idf.org/guidelines/type-2-diabetes Accessed 30 December 2012
-
- Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med 2005;118:422–425 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

