HIV/AIDS eradication
- PMID: 23735743
- PMCID: PMC3714230
- DOI: 10.1016/j.bmcl.2013.05.032
HIV/AIDS eradication
Abstract
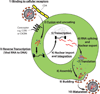
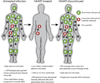
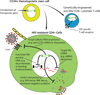
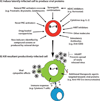
Antiretroviral therapy can inhibit HIV replication in patients and prevent progression to AIDS. However, it is not curative. Here we provide an overview of what antiretroviral drugs do and how the virus persists during therapy in rare reservoirs, such as latently infected CD4+ T cells. We also outline several innovative methods that are currently under development to eradicate HIV from infected individuals. These strategies include gene therapy approaches intended to create an HIV-resistant immune system, and activation/elimination approaches directed towards flushing out latent virus. This latter approach could involve the use of novel chemically synthesized analogs of natural activating agents.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

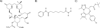
References
-
- UNAIDS. UNAIDS Report on the Global AIDS Epidemic. 2012
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. N Engl J Med. 2009:361–2209. - PubMed
-
- Moir S, Chun TW, Fauci AS. Annu Rev Pathol. 2011:6–223. - PubMed
-
- Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, Richman DD, Rizzardini G, Thomas DL, Jacobsen DM, Volberding PA. JAMA. 2012:308–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

