Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity
- PMID: 23736036
- PMCID: PMC3694243
- DOI: 10.1038/bjc.2013.262
Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity
Abstract
Background: Fluoropyrimidine drugs are extensively used for the treatment of solid cancers. However, adverse drug reactions are a major clinical problem, often necessitating treatment discontinuation. The aim of this study was to identify pharmacogenetic markers predicting fluoropyrimidine toxicity.
Methods: Toxicity in the first four cycles of 5-fluorouracil or capecitabine-based chemotherapy were recorded for a series of 430 patients. The association between demographic variables, DPYD, DPYS, TYMS, MTHFR, CDA genotypes, and toxicity were analysed using logistic regression models.
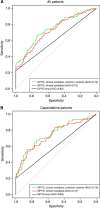
Results: Four DPYD sequence variants (c.1905+1G>A, c.2846A>T, c.1601G>A and c.1679T>G) were found in 6% of the cohort and were significantly associated with grade 3-4 toxicity (P<0.0001). The TYMS 3'-untranslated region del/del genotype substantially increased the risk of severe toxicity (P=0.0123, odds ratio (OR)=3.08, 95% confidence interval (CI): 1.38-6.87). For patients treated with capecitabine, a MTHFR c.1298CC homozygous variant genotype predicted hand-foot syndrome (P=4.1 × 10⁻⁶, OR=9.99, 95% CI: 3.84-27.8). The linked CDA c.-92A>G and CDA c.-451C>T variants predicted grade 2-4 diarrhoea (P=0.0055, OR=2.3, 95% CI: 1.3-4.2 and P=0.0082, OR=2.3, 95% CI: 1.3-4.2, respectively).
Conclusion: We have identified a panel of clinically useful pharmacogenetic markers predicting toxicity to fluoropyrimidine therapy. Dose reduction should be considered in patients carrying these sequence variants.
Figures
Similar articles
-
Pretreatment serum uracil concentration as a predictor of severe and fatal fluoropyrimidine-associated toxicity.Br J Cancer. 2017 May 23;116(11):1415-1424. doi: 10.1038/bjc.2017.94. Epub 2017 Apr 20. Br J Cancer. 2017. PMID: 28427087 Free PMC article.
-
Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data.Lancet Oncol. 2015 Dec;16(16):1639-50. doi: 10.1016/S1470-2045(15)00286-7. Epub 2015 Oct 23. Lancet Oncol. 2015. PMID: 26603945
-
DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis.Lancet Oncol. 2018 Nov;19(11):1459-1467. doi: 10.1016/S1470-2045(18)30686-7. Epub 2018 Oct 19. Lancet Oncol. 2018. PMID: 30348537
-
The role of pharmacogenetics in capecitabine efficacy and toxicity.Cancer Treat Rev. 2016 Nov;50:9-22. doi: 10.1016/j.ctrv.2016.08.001. Epub 2016 Aug 10. Cancer Treat Rev. 2016. PMID: 27569869 Review.
-
Evaluation of 5-fluorouracil degradation rate and Pharmacogenetic profiling to predict toxicity following adjuvant Capecitabine.Eur J Clin Pharmacol. 2017 Feb;73(2):157-164. doi: 10.1007/s00228-016-2160-8. Epub 2016 Nov 18. Eur J Clin Pharmacol. 2017. PMID: 27864592
Cited by
-
Association of Single-Nucleotide Polymorphisms in Capecitabine Bioactivation Pathway with Adjuvant Therapy Safety in Colorectal Cancer Patients.Pharmaceutics. 2023 Oct 28;15(11):2548. doi: 10.3390/pharmaceutics15112548. Pharmaceutics. 2023. PMID: 38004528 Free PMC article.
-
Severe toxicity to capecitabine due to a new variant at a donor splicing site in the dihydropyrimidine dehydrogenase (DPYD) gene.Cancer Manag Res. 2018 Oct 11;10:4517-4522. doi: 10.2147/CMAR.S174470. eCollection 2018. Cancer Manag Res. 2018. PMID: 30349384 Free PMC article.
-
Improving single nucleotide polymorphisms genotyping accuracy for dihydropyrimidine dehydrogenase testing in pharmacogenetics.Explor Target Antitumor Ther. 2024;5(2):374-383. doi: 10.37349/etat.2024.00223. Epub 2024 Apr 24. Explor Target Antitumor Ther. 2024. PMID: 38745766 Free PMC article.
-
The Role of Dihydropyrimidine Dehydrogenase and Thymidylate Synthase Polymorphisms in Fluoropyrimidine-Based Cancer Chemotherapy in an Iranian Population.Avicenna J Med Biotechnol. 2020 Jul-Sep;12(3):157-164. Avicenna J Med Biotechnol. 2020. PMID: 32695278 Free PMC article.
-
Alternative chemoradiotherapy in anal carcinoma patients with mutations in thymidylate synthase and dihydropyrimidine dehydrogenase genes.Therap Adv Gastroenterol. 2021 Jul 3;14:17562848211024464. doi: 10.1177/17562848211024464. eCollection 2021. Therap Adv Gastroenterol. 2021. PMID: 34276810 Free PMC article.
References
-
- Afzal S, Jensen SA, Vainer B, Vogel U, Matsen JP, Sorensen JB, Andersen PK, Poulsen HE. MTHFR polymorphisms and 5-FU-based adjuvant chemotherapy in colorectal cancer. Ann Oncol. 2009;20 (10:1660–1666. - PubMed
-
- Amstutz U, Farese S, Aebi S, Largiader CR. Dihydropyrimidine dehydrogenase gene variation and severe 5-fluorouracil toxicity: a haplotype assessment. Pharmacogenomics. 2009;10 (6:931–944. - PubMed
-
- Amstutz U, Froehlich TK, Largiader CR. Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity. Pharmacogenomics. 2011;12 (9:1321–1336. - PubMed
-
- Braun MS, Richman SD, Thompson L, Daly CL, Meade AM, Adlard JW, Allan JM, Parmar MK, Quirke P, Seymour MT. Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial. J Clin Oncol. 2009;27 (33:5519–5528. - PubMed
-
- Cancer M-AGI Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. J Clin Oncol. 1998;16:3537–3541. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

